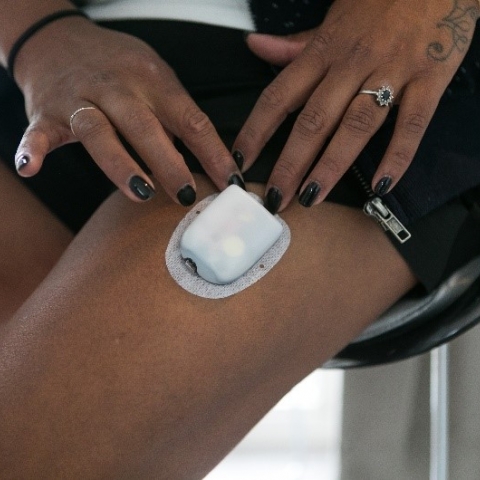
Insulet paid a fee to engage Ariana as a content creator and has an ongoing commercial relationship with Ariana as a Sponsored Omnipod user however the views expressed in this testimonial are solely those of Ariana.
Select Region
- United States
- Estados Unidos - Español
- Australia
- België - Nederlands
- Belgique - Français
- Canada - English
- Canada - Français
- Croatia - English
- Danmark - Dansk
- Denmark - English
- Deutschland
- España - Español
- Finland - English
- Suomi - Suomalainen
- France
- Greece - English
- Iceland - English
- Italia
- Israel - English
- Kuwait - English
- Nederland
- Norge - Norsk
- Norway - English
- Österreich
- Qatar - English
- المملكة العربية السعودية - عربي
- Saudi Arabia - English
- Schweiz - Deutsch
- Suisse - Français
- Svizzero - Italiano
- Sverige - Svenska
- Sweden - English
- Turkey - English
- United Arab Emirates - English
- United Kingdom
- Other