All About Omnipod® 5 Automated Insulin Delivery (AID) System
It’s official, the Omnipod® 5 Automated Insulin Delivery (AID) System has been cleared by the Food and Drug Administration (FDA) for individuals ages 6 years and older with type 1 diabetes!
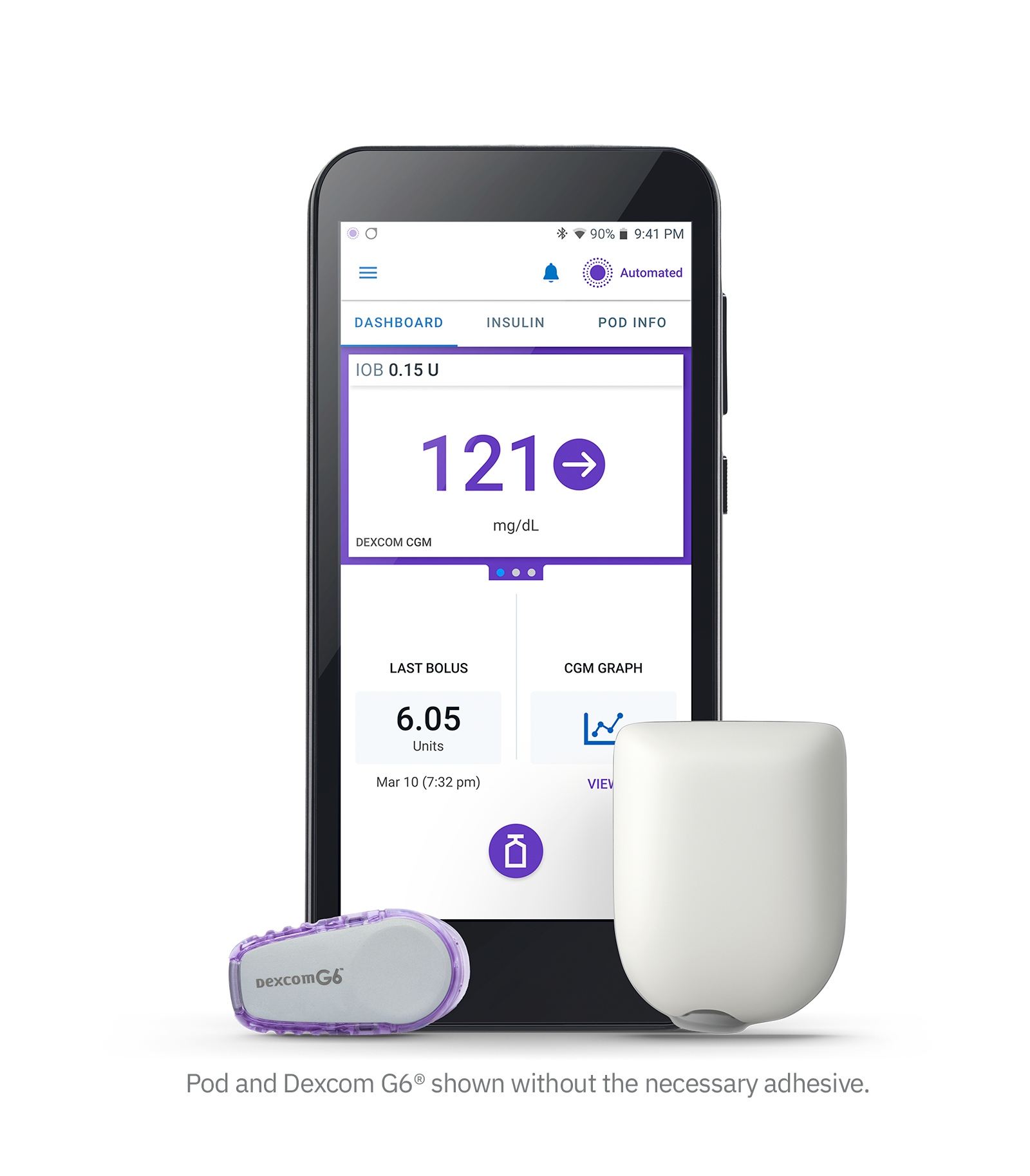
Omnipod 5 is the first tubeless AID system managed through a compatible smartphone1 in the U.S. and the only tubeless AID system that integrates with the Dexcom G6® Continuous Glucose Monitor (CGM) System2 to automatically adjust insulin delivery and manage glucose levels both day and night, helping to protect against highs and lows3. Like Omnipod DASH® it continues to be wearable and waterproof†.
The specifics
Omnipod 5 is designed to make it easier than ever to manage glucose with no multiple daily injections, tubing, or fingersticks4 to help simplify life with diabetes.
The Omnipod 5 System consists of:
- the tubeless Pod enhanced with SmartAdjust™ technology
- the Dexcom G6® CGM (not included)
- and the Omnipod 5 app with its integrated SmartBolus Calculator. The app can be downloaded onto a compatible personal smartphone and is also integrated into the Omnipod 5 Controller, which is provided free with the first prescription.
Every five minutes, with our SmartAdjust technology, the Pod receives a CGM value and trend, and predicts where glucose will be 60 minutes into the future. The system then increases, decreases, or pauses insulin delivery using the user’s desired and customized glucose target, helping to protect against highs and lows5.
Access
Unlike traditional insulin pumps, Omnipod 5 will be available through a customer’s pharmacy6 benefit which comes with the convenience of picking up their Pods just as they would their insulin and other diabetes supplies, and the benefits of no contract, no commitment, and no obligation.
We’ll now start a Limited Market Release (LMR), the length of which is determined by achieving metrics on simplicity, access and commercial readiness to ensure a best-in-class product experience for our Podders®. During LMR, the system will be used by an already predetermined number of people living with diabetes. The great news is, Omnipod 5 is expected to be broadly available shortly after the Limited Market Release.
In the meantime
People with diabetes can enjoy the benefits of Pod Therapy today through OmnipodPromise™, which allows new and existing users to start on Omnipod DASH® and upgrade to Omnipod 5 at no additional cost when product is available7.
__________
Don’t wait to experience Pod therapy, get started on Omnipod DASH® today with a 30-day free trial*.