Omnipod® 5 FAQs:
About Omnipod 5
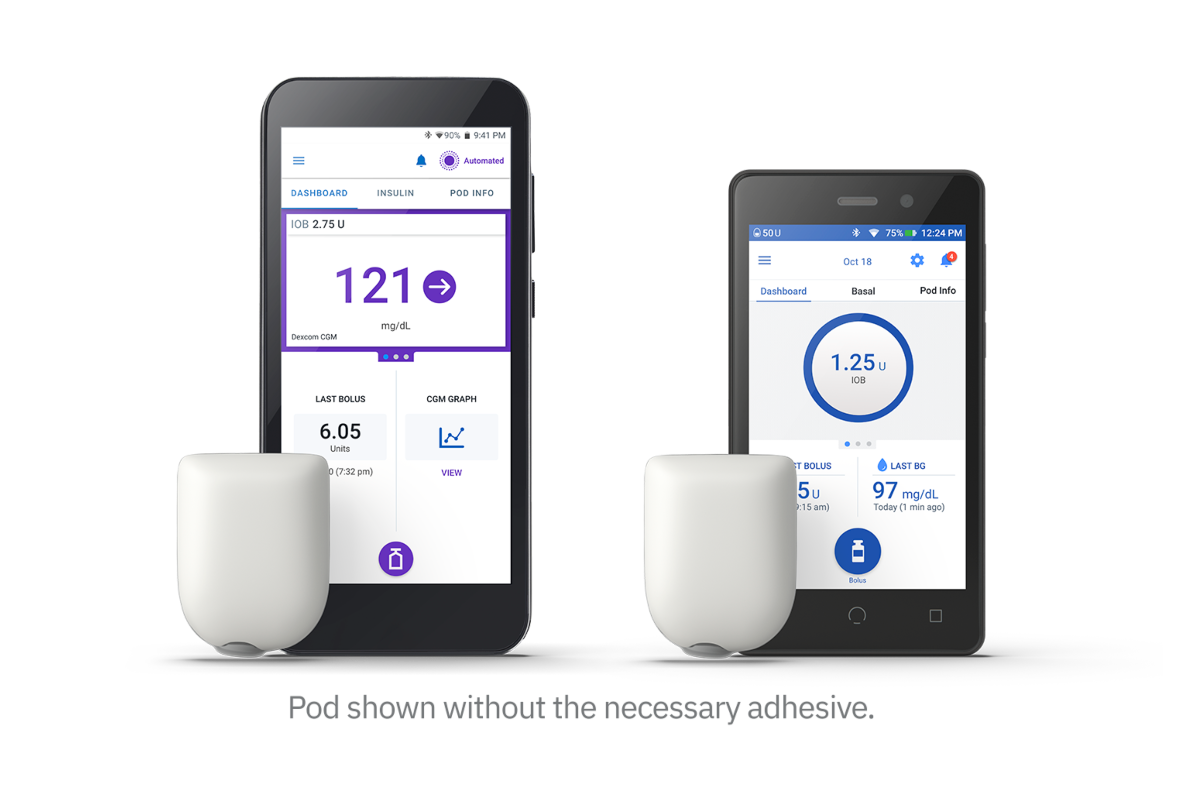
Omnipod® 5 is the only tubeless automated insulin delivery system integrated with multiple CGM sensors for proactive glucose control without multiple daily injections.
Below are some answers to commonly asked questions about Omnipod 5.
What is the Omnipod 5 Automated Insulin Delivery System?
What continuous glucose monitoring devices are compatible with the Omnipod 5 Automated Insulin Delivery System?
What phone(s) is the Omnipod 5 Automated Insulin Delivery System compatible with?
What is the age range indicated for the Omnipod 5 System? Is the Omnipod 5 System intended for anyone with insulin requiring Diabetes?
Will the Omnipod DASH® Pods work with the Omnipod® 5 Automated Insulin Delivery System?
Is the Omnipod 5 Automated Insulin Delivery System an Artificial Pancreas?
How does Automated Mode differ from Manual Mode?
What is the difference between the Omnipod 5 System and Omnipod DASH?
What languages are available in the Omnipod 5 System?
Do I still have to change the Omnipod 5 Pod every three days?
Is the Omnipod 5 Pod the same size as previous Omnipod Pods?
My prescription for Omnipod 5 has not changed, but my Pods look different. Is this the same Pod?
What data management system is Omnipod 5 compatible with?
What accessories are available for Omnipod 5?
Looking for more?


Contact Us
Podders can reach out to our Customer Care team using the Contact Us form or by calling 1‑800‑591‑3455. We’re available 24/7.

Is Omnipod right for me?
Meet our family of tubeless wireless insulin delivery devices.


Omnipod Reviews
What's it like to be a Podder? Check out real stories of how Omnipod has simplified lives.
Still have questions about Omnipod 5?
Click here to access the main FAQ page.
2. Sherr JL, et al. Diabetes Care (2022). Study in 80 people with T1D aged 2 - 5.9 yrs involving 2 weeks standard diabetes therapy followed by 3 months Omnipod 5 use in Automated Mode. Average time with high blood glucose in very young children, standard therapy vs 3-month Omnipod 5: 39.4% vs. 29.5%. Average time with low blood glucose in very young children, standard therapy vs 3-month Omnipod 5: 3.43% vs. 2.46%.
3. Pasquel FJ, et al. JAMA Network Open (2025). Prospective pivotal trial in 305 participants with T2D aged 18-75 yrs. Study included a 14-day standard therapy (ST) phase followed by a 13-week Omnipod 5 hybrid closed-loop phase. Mean time >180 mg/dL as measured by CGM: ST = 54%, 3-mo Omnipod 5 = 34%, P<0.001. Mean time <70 mg/dL as measured by CGM: ST = 0.2%, 3-mo Omnipod 5 = 0.2%.
The Omnipod® 5 Automated Insulin Delivery System is indicated for use by individuals with type 1 diabetes mellitus in persons 2 years of age and older and type 2 diabetes mellitus in persons 18 years of age and older. The Omnipod 5 System is intended for single patient, home use and requires a prescription. The Omnipod 5 System is compatible with the following U-100 insulins: NovoLog®, Humalog®, and Admelog®.
Available products subject to current insurance coverage and product indication for use. Insulet can only support onboarding for those customers within the product indication.
Refer to the Omnipod 5 Automated Insulin Delivery System User Guide and www.omnipod.com/safety for complete safety information including indications, contraindications, warnings, cautions, and instructions. Warning: DO NOT start to use the Omnipod 5 System or change settings without adequate training and guidance from a healthcare provider. Initiating and adjusting settings incorrectly can result in over-delivery or under-delivery of insulin, which could lead to hypoglycemia or hyperglycemia. WARNING: SmartAdjust technology should NOT be used by anyone under the age of 2 years old. SmartAdjust technology should also NOT be used in people who require less than 5 units of insulin per day as the safety of the technology has not been evaluated in this population.
The Omnipod DASH® Insulin Management System is intended for subcutaneous delivery of insulin at set and variable rates for the management of diabetes mellitus in persons requiring insulin. Additionally, the Omnipod DASH System is interoperable with a compatible blood glucose meter to receive and display blood glucose measurements. The Omnipod DASH System has been tested and found to be safe for use with the following U-100 insulin: NovoLog®, Humalog®, Apidra®, Fiasp®, Lyumjev®, or Admelog®.
Available products subject to current insurance coverage and product indication for use. Insulet can only support onboarding for those customers within the product indication. Omnipod DASH is currently indicated for all users with insulin-requiring diabetes. Feel free to contact your doctor regarding all products that might be right for you.
Refer to the Omnipod DASH Insulin Management System User Guide at omnipod.com for complete safety information including indications, contraindications, warnings, cautions, and instructions.