How Omnipod® 5 Simplifies Life for Tom of Type One Talks


“With an automated insulin delivery system like Omnipod 5, I get less alerts from my CGM and I sleep better. I wake up with an [in range] blood sugar almost every day.1-3 It’s a game changer.”
Tom Vesely, Sponsored Podder®
Individual results may vary.
- Eliminates the need for multiple daily injections
- Shown to lower A1C1-3
- Tube-free
- For people with type 1 and type 2 diabetes*
Automated insulin delivery with the help of your CGM sensor
Omnipod 5 provides up to 3 days (72 hours) of continuous insulin delivery without the need for multiple daily injections. With the help of your CGM sensor, the System increases, decreases, or pauses insulin delivery every 5 minutes based on your CGM value and trend to help keep glucose levels in range.1-3
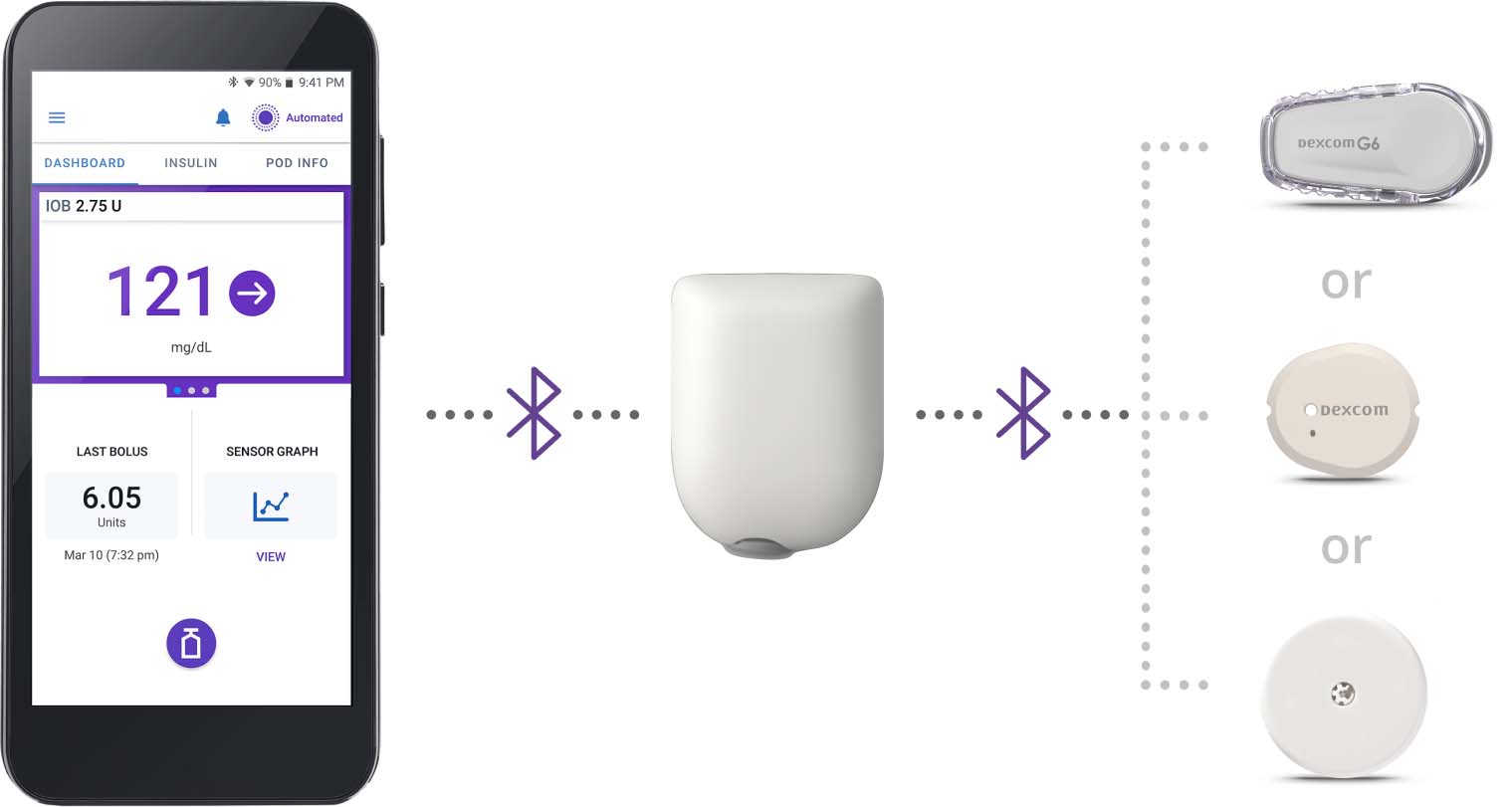

Omnipod 5 Controller or App
Take control of the entire System from a compatible smartphone or use the Controller included with your first prescription. Deliver insulin for meals, change your Pod, and more with just a few taps on the Omnipod 5 App.
Pod
Tubeless, wearable and waterproof.‡ The Pod sits right on your body and provides continuous insulin delivery for up to 3 days (72 hours).
CGM sensor
Your Continuous Glucose Monitor (CGM) sensor§ sends glucose values and trends to the Pod to help the System make automated insulin delivery decisions.
Omnipod 5 pairs with the Dexcom G6, Dexcom G7, and FreeStyle Libre 2 Plus Sensor.
The #1 Prescribed Automated Insulin Delivery System◊
USA 2024, Data on file◊
The advantage of a patch pump like Omnipod 5 is that it stays connected to your body all the time. You can shower with it, you can swim with it,‡ and the algorithm keeps working in the background giving you the right amount of insulin trying to keep you in the optimal range.1-3


Get started today
Omnipod 5 is available exclusively through the pharmacy, which means you or your loved one can make the switch to Omnipod 5 from multiple daily injections, a tubed pump, or even another Omnipod product at almost any time as your coverage and approvals allow.
Most Omnipod 5 users pay $30
or less per month#


The support you need to succeed
With Omnipod, you get more than just an insulin management system, you also get:
- A training session tailored to your needs
- Access to a 24/7 support team
- A community of Podders just like you, sharing tips and experiences for simplifying life with diabetes
Get Started Today!
Choose a path below and answer a few questions to find out which Omnipod product is best for you. It’s that easy.
I’d like to check my coverage
Find out your estimated copay
I'd like a free trial of Omnipod††
Take the Pod for a test drive
1. Brown et al. Diabetes Care (2021). Study in 240 people with T1D aged 6 - 70 years involving 2 weeks standard diabetes therapy followed by 3 months Omnipod 5 use in Automated Mode. Average time (6AM-12AM) in Target Glucose range (from CGM) in adults/adolescents and children for standard therapy vs Omnipod 5 = 64.8% vs. 72.5%; 51.5% vs. 64.6%. Average time (12AM-6AM) in Target Glucose range (from CGM) in adults/adolescents and children for standard therapy vs Omnipod 5 = 64.3% vs. 78.1%; 55.3% vs. 78.1%. Average A1c in adults/adolescents and children, standard therapy vs. Omnipod 5 = 7.16% vs 6.78%; 7.67% vs 6.99%. Study funded by Insulet.
2. Sherr JL, et al. Diabetes Care (2022). Study in 80 people with T1D aged 2 - 5.9 yrs involving 2 weeks standard diabetes therapy followed by 3 months Omnipod 5 use in Automated Mode. Average time in Target Glucose range (6AM-12AM) from CGM in standard therapy vs Omnipod 5 = 56.9% vs. 63.7%. Average time in Target Glucose range (12AM-6AM) from CGM in standard therapy vs. Omnipod 5 = 58.2% vs 81.0%. Average A1c in standard therapy vs. Omnipod 5 = 7.4% vs. 6.9%. Study funded by Insulet.
3. Pasquel FJ, et al. JAMA Network Open (2025). Prospective pivotal trial in 305 participants with T2D aged 18-75 yrs. Study included a 14-day standard therapy (ST) phase followed by a 13-week Omnipod 5 hybrid closed-loop phase. Mean overnight time 70-180 mg/dL (12AM-6AM) as measured by CGM: ST = 49%, 3-mo Omnipod 5 = 70%. Mean daytime 70-180 mg/dL (6AM-12AM) as measured by CGM: ST = 44%, 3-mo Omnipod 5 = 64%. Statistical testing not done to assess significance of change between ST phase and Omnipod 5 System phase. Mean HbA1c: ST vs. 13-week Omnipod 5: 8.2% vs. 7.4%, P<0.001.
‡The Pod has an IP28 rating for up to 25 feet for 60 minutes. The Omnipod 5 Controller is not waterproof.
§CGM sensors sold separately. The Dexcom G6 and G7 must be used with the Dexcom mobile app to use automated mode. The Dexcom receiver is not compatible.
#Majority defined as at least 50% of patient co-pays $30 or less per month. Among All Paid Omnipod 5 G7G6 Pods Commercial and Medicare Claims from January 2024 to December 2024. Includes benefits and offerings available through Insulet, such as the copay card program. Actual co-pay amount depends on patient’s health plan and coverage, they may fluctuate and be higher or lower than the advertised amount on a monthly basis. Source: IQVIA OPC Library
††Omnipod DASH® 30-Day Free Trial
Terms and Conditions
1. Program Eligibility
Eligibility criteria: Subject to program limitations and terms and conditions, the Omnipod DASH 30-day Free Trial Program (the “Program”) is open to patients who have a valid Omnipod DASH prescription and who have commercial or private insurance, including plans available through state and federal healthcare exchanges. The Program is open only to new Pod Therapy patients coming from multiple daily injections or tubed pumps only who have not previously used Omnipod® 5, Omnipod DASH or Omnipod Insulin Management System.
This offer is not valid for participants whose Omnipod DASH prescription is paid for in whole or in part by Medicare, Medicaid, or any other federal or state programs, or where prohibited by law. Participants on certain commercial insurance plans may not be eligible. This offer is only valid in the United States, Puerto Rico, and the U.S. territories. Please contact Insulet Customer Support at 1-800-591-3455 for details.
2. Program Details
With this program, Participants may be eligible to receive a 30-day supply of Omnipod DASH products at no cost for them. Eligible participants have two (2) options, based on the following:
• A participant shall sign the 30-day Omnipod DASH Free Trial Acknowledgement through the appropriate platform provided by Insulet’s pharmacy partner.
• Once Insulet, or its pharmacy partner, has received the request, including a valid prescription for the Omnipod DASH Intro Kit and Omnipod DASH Pods, the participant’s benefits will be checked by Insulet or its pharmacy partner.
• If the benefits check results in a monthly copay equal to or below two hundred dollars ($200), then Insulet, or its pharmacy partner, shall issue a one-time only copay card to the participant, for a value equal to the out-of-pocket expenses the participant would have to pay for an Omnipod DASH 30-day initial shipment, in accordance with Section 3, below.
• If the benefits check result in a copay greater than two hundred dollars ($200), Insulet, or its authorized partners, shall arrange for the shipment of one (1) Omnipod DASH Intro Kit, in accordance with Section 4, below.
• For the purpose of clarity, the term “copay” shall encompass any out-of-pocket expense for one (1) month’s supply of Pods, including any deductible, copays and other out-of-pocket expenses that the participant would have to disburse to procure said supply of Pods.
• Any copay assistance may not apply to a participant’s health plan’s deductible if prohibited by state law or by a health plan.
Insulet reserves the right to change, amend or rescind this Program, in whole or in part, at any time.
3. Copay Card
Should participant be deemed eligible to receive an Omnipod DASH Copay Card, participant shall receive electronically one (1) Omnipod DASH Copay Card, valid for a single use, in the amount required for the participant to procure a 30-day supply of Omnipod DASH Pods at a participating pharmacy. In addition, Insulet’s pharmacy partners shall ship, at no cost to participant, one (1) Omnipod DASH Starter Kit, which shall include:
- One (1) Omnipod DASH Personal Diabetes Manager (PDM)
- One (1) Omnipod DASH User Guide
- One (1) PDM charging cable
The Omnipod DASH Starter Kit shall be delivered to the shipping address indicated by participant in their Acknowledgment Form. Any estimate date of delivery is given solely for participant’s information and does not constitute a warranty that the Starter Kit will be delivered on said date. Participant is responsible to provide an accurate delivery address, to receive shipment of the Starter Kit and to verify the content of the Starter Kit.
4. Product Dispense
Should participant be deemed eligible to receive a one-time dispense of Omnipod DASH Pods at no cost to them, Insulet, or its authorized partner, shall dispense one (1) Omnipod DASH Intro Kit, which shall include:
- One (1) Omnipod DASH PDM
- Eleven (11) Omnipod DASH Pods
- One (1) Omnipod DASH User Guide
- One (1) PDM charging cable
The Omnipod DASH Intro Kit shall be delivered to the shipping address indicated by participant in their Acknowledgment Form. Any estimate date of delivery is given solely for participant’s information and does not constitute a warranty that the Intro Kit will be delivered on said date. Participant is responsible to provide an accurate delivery address, to receive shipment of the Intro Kit and to verify the content of the Intro Kit.
††Omnipod® 5 Intro Kit 30-Day Trial
Terms and Conditions
1. Program Eligibility
Eligibility criteria: Subject to program limitations and terms and conditions, the Omnipod 5 Intro Kit 30-day trial program (the “Program”) is open to patients who have a valid Omnipod 5 prescription as well as a compatible CGM prescription and who have commercial or private insurance, including plans available through state and federal healthcare exchanges. In order to be eligible, the patient’s eligible insurance plan must include coverage for Omnipod 5 Pods. The Program is open to new Pod Therapy patients coming from multiple daily injections or tubed pumps only who have not previously used Omnipod 5, Omnipod DASH® or Omnipod Insulin Management System.
This offer is not valid for participants whose Omnipod 5 or compatible CGM prescription is paid for in whole or in part by Medicare, Medicaid, or any other federal or state programs. It is not valid for cash-paying participants or where prohibited by law. A participant is considered cash-paying where the participant has no insurance coverage for Omnipod 5 or where the participant has commercial or private insurance but Insulet determines in its sole discretion the participant is effectively uninsured because such coverage does not provide a material level of financial assistance for the cost of an Omnipod 5 prescription. Participants on certain commercial insurance plans may not be eligible. This offer is only valid in the United States, Puerto Rico, and the U.S. territories. Participants receiving their products through the Durable Medical Equipment or Pharmacy Durable Medical Equipment channel are not eligible to participate in the copay card program. Please contact Insulet Customer Support at 1-800-591-3455 for details.
2. Program Details
With this program, Participants may be eligible to receive a limited supply of Omnipod 5 products at no cost for them. Eligible participants have two (2) options, based on the following:
• A participant shall sign the Omnipod® 5 Intro Kit 30-Day Trial Acknowledgement through the appropriate platform provided by Insulet.
• Once Insulet has received the request, the request shall be escalated to Insulet’s pharmacy partner, where a request for a prescription shall be sent to the participant’s healthcare professional. If a valid prescription is received, both for the Omnipod 5 Intro Kit and the Omnipod 5 Pods, the participant’s benefits will be checked by Insulet or its partners.
• IF the benefits check results in a monthly copay equal to or below two hundred dollars ($200), then Insulet shall issue a one-time only copay card to the participant, for a value equal to the out-of-pocket expenses the participant would have to pay for an Omnipod 5 Intro Kit, in accordance with Section 3, below.
• IF the benefits check result in a copay greater than two hundred dollars ($200), Insulet, or its authorized partners, shall arrange for the shipment of one (1) Omnipod 5 Intro Kit, in accordance with Section 4, below.
• For the purpose of clarity, the term “copay” shall encompass any out-of-pocket expense for one (1) month’s supply of Pods, including any deductible, copays and other out-of-pocket expenses that the participant would have to disburse to procure said supply of Pods.
• Any copay assistance may not apply to a participant’s health plan’s deductible if prohibited by state law or by a health plan.
• In order to use the Omnipod 5 System in Automated Mode, the User must also procure a compatible CGM. This program does not include supply of a compatible CGM.
Insulet reserves the right to change, amend or rescind this Program, in whole or in part, at any time.
3. Copay Card
Should participant be deemed eligible to receive an Omnipod 5 Copay Card, participant shall receive electronically one (1) Omnipod 5 Copay Card, valid for a single use, in the amount required for the participant to procure one (1) Omnipod 5 Intro Kit, which shall include:
- One (1) Omnipod 5 Controller
- Ten (10) Omnipod 5 Pods
- One (1) Omnipod 5 User Guide
- One (1) Controller charging cable
4. Product Dispense
Should participant be deemed eligible to receive a one-time dispense of Omnipod 5 Pods at no cost to them, Insulet, or its authorized partner, shall dispense one (1) Omnipod 5 Intro Kit, which shall include:
- One (1) Omnipod 5 Controller
- Ten (10) Omnipod 5 Pods
- One (1) Omnipod 5 User Guide
- One (1) Controller charging cable
The Omnipod 5 Intro Kit shall be delivered to the shipping address indicated by participant in their Acknowledgment Form. Any estimate date of delivery is given solely for participant’s information and does not constitute a warranty that the Intro Kit will be delivered on said date. Participant is responsible to provide an accurate delivery address, to receive shipment of the Intro Kit and to verify the content of the Intro Kit.
*Risk Statement
The Omnipod 5 Automated Insulin Delivery System is indicated for use by individuals with type 1 diabetes mellitus in persons 2 years of age and older and type 2 diabetes mellitus in persons 18 years of age and older.
The Omnipod 5 System is intended for single patient, home use and requires a prescription. The Omnipod 5 System is compatible with the following U-100 insulins: NovoLog®, Humalog®, and Admelog®.
The Omnipod 5 ACE Pump (Pod) is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in persons requiring insulin. The Omnipod 5 ACE Pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices.
SmartAdjust™ technology is intended for use with compatible integrated continuous glucose monitors (iCGM) and alternate controller enabled (ACE) pumps to automatically increase, decrease, and pause delivery of insulin based on current and predicted glucose values.
The Omnipod 5 SmartBolus Calculator is intended to calculate a suggested bolus dose based on user-entered carbohydrates, most recent sensor glucose value (or blood glucose reading if using fingerstick), rate of change of the sensor glucose (if applicable), insulin on board (IOB), and programmable correction factor, insulin to carbohydrate ratio, and target glucose value.
WARNING: SmartAdjust technology should NOT be used by anyone under the age of 2 years old. SmartAdjust technology should also NOT be used in people who require less than 5 units of insulin per day as the safety of the technology has not been evaluated in this population.
The Omnipod 5 System is NOT recommended for people who are unable to monitor glucose as recommended by their healthcare provider, are unable to maintain contact with their healthcare provider, are unable to use the Omnipod 5 System according to instructions, are taking hydroxyurea as it could lead to falsely elevated CGM values and result in over-delivery of insulin that can lead to severe hypoglycemia, and do NOT have adequate hearing and/or vision to allow recognition of all functions of the Omnipod 5 System, including alerts, alarms, and reminders. Device components including the Pod, CGM transmitter, and CGM sensor must be removed before Magnetic Resonance Imaging (MRI), Computed Tomography (CT) scan, or diathermy treatment. In addition, the Controller and smartphone should be placed outside of the procedure room. Exposure to MRI, CT, or diathermy treatment can damage the components. Visit www.omnipod.com/safety for additional important safety information.
WARNING: DO NOT start to use the Omnipod 5 System or change settings without adequate training and guidance from a healthcare provider. Initiating and adjusting settings incorrectly can result in over-delivery or under-delivery of insulin, which could lead to hypoglycemia or hyperglycemia.