Prescribing Omnipod DASH®


Continuous insulin delivery for up to 3 days (72 hours)
- Proven results
- Simple to use
- Covered through the pharmacy
Prescribe Omnipod DASH®:
How to prescribe the Omnipod DASH® Insulin Management System


Step 1 (Pods)
Prescribe the Omnipod DASH® Pods directly to your patient’s pharmacy
Omnipod DASH® Pods (Gen 4) Refill 5-Pack
Product NDC: 08508-2000-05
Quantity fill: 2 boxes (30-day supply)
Refill: 30 days
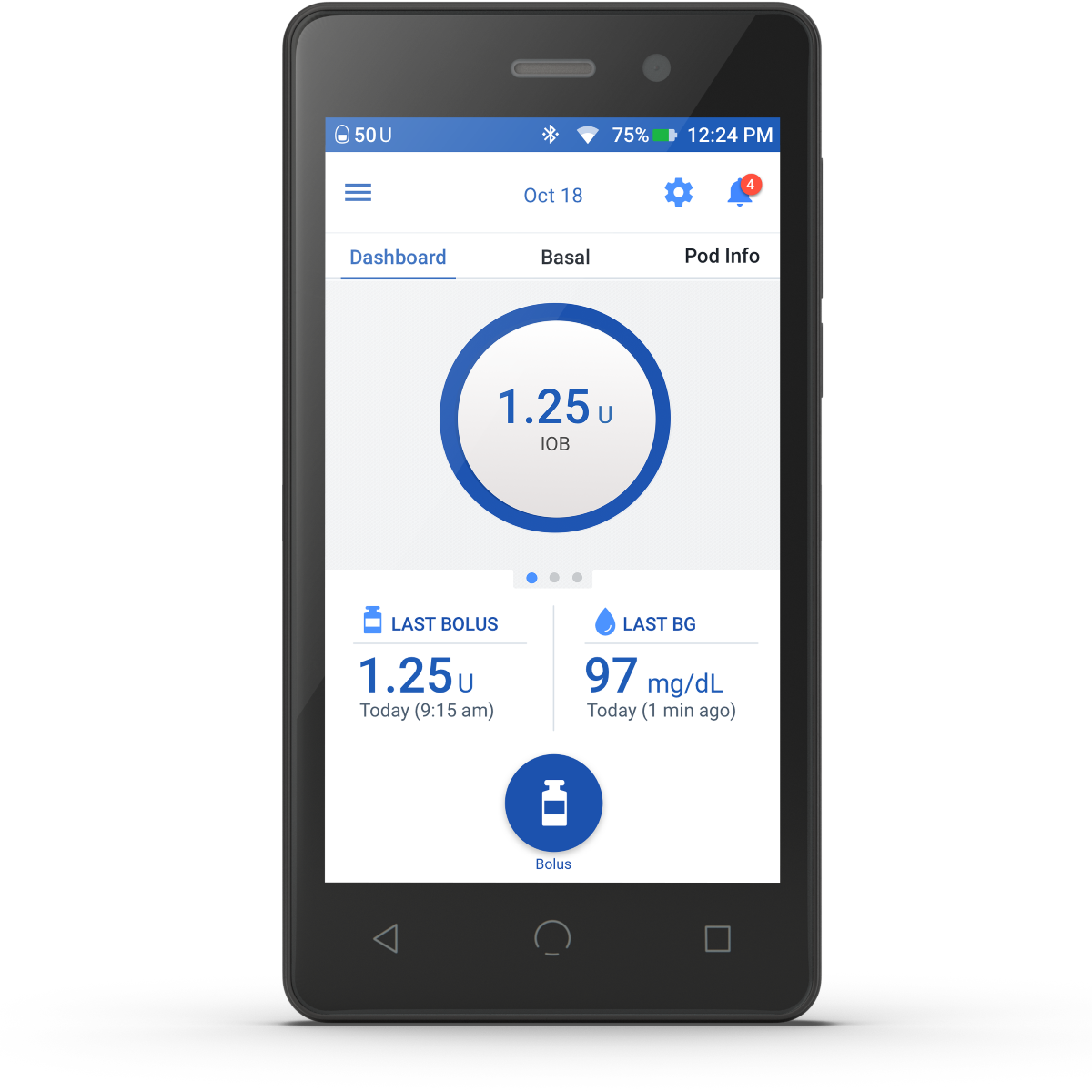

Step 2 (PDM)
Once patient has received their Omnipod DASH® Pods, prescribe the Omnipod DASH® PDM electronically to:
ASPN Pharmacies, LLC
290 West Mount Pleasant Ave
Building 2, 4th Floor, Suite 2400
Livingston, NJ 07039
Omnipod DASH® PDM (Gen 4) Kit*
Product NDC: 08508-2000-00
Quantity fill: 1 PDM Kit
Refill: 0
Important Safety Information: The Omnipod DASH® Insulin Management System is intended for subcutaneous delivery of insulin at set and variable rates for the management of diabetes mellitus in persons requiring insulin. Additionally, the Omnipod DASH® System is interoperable with a compatible blood glucose meter to receive and display blood glucose measurements. The Omnipod DASH® System has been tested and found to be safe for use with the following U-100 insulin: NovoLog®, Humalog®, Apidra®, Fiasp®, Lyumjev®, or Admelog®. Refer to the Omnipod DASH® Insulin Management System User Guide at omnipod.com for complete safety information including indications, contraindications, warnings, cautions, and instructions.
Financial Assistance Programs May Be Available
Have your patients call customer care at 1-800-591-3455 to learn more.
INS-ODS-04-2023-00005 v 2.0