Omnipod® 5 Real‑World Evidence
"Healthcare professionals may reasonably suggest more stringent A1c goals ... if they can be achieved without significant hypoglycemia."2
American Diabetes Association Standards of Care in Diabetes, 2024
The real-world evidence showed that Omnipod® 5 users achieved nearly 70% Time in Range with only 1.12% of time spent in hypoglycemia at an average target of 110 mg/dL.3
Highlights at a Glance
AID All Day Long
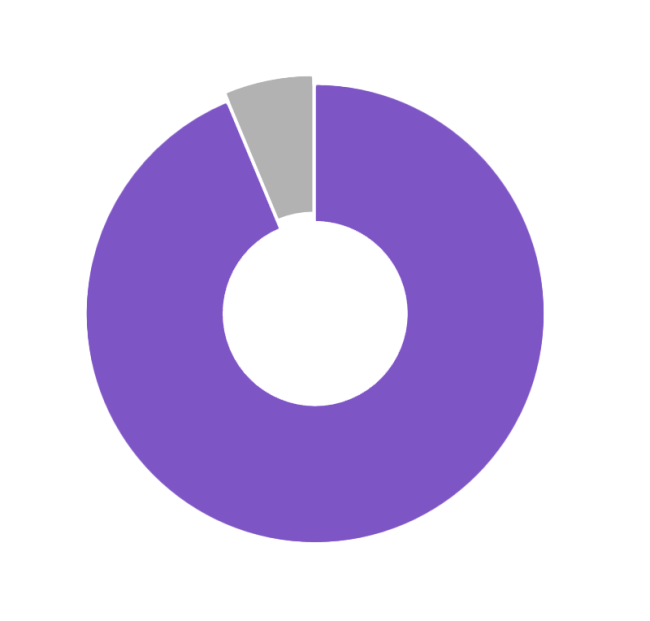
Patients Spent 94% of Time in Automated Mode4
- Nearly 70,000 Omnipod 5 users with type 1 diabetes representative of a diverse population, including nearly 20% of those who transition from MDI.5
- Nearly 70% TIR at an average target of 110 mg/dL.6
- Users on average spent less than 1% of time below range.7
- There is no need to disconnect from therapy. Patients spent nearly 94% of the time in Automated Mode8
- Patients previously on MDI achieved 70.8% TIR at an average target of 110 mg/dL.9
- Automatic upload of data from Omnipod 5 users minimizes selection bias.10
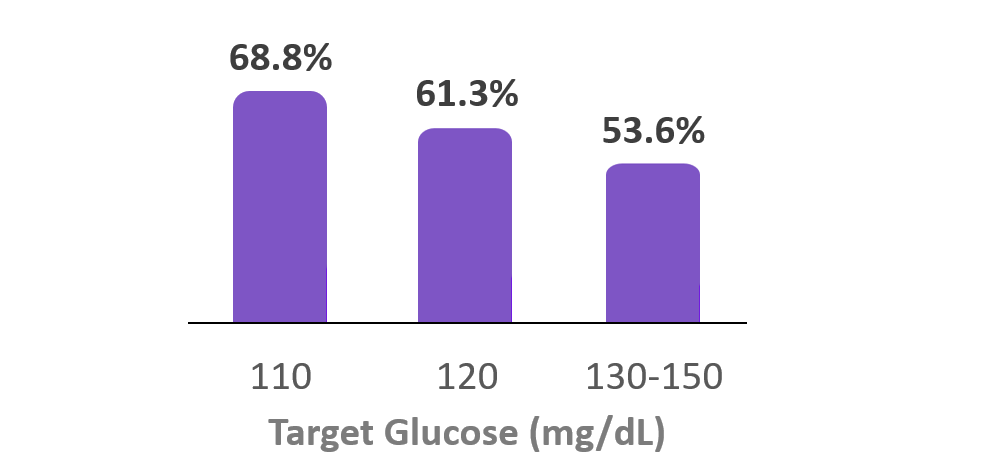
Time in Range
(70-180 mg/dL)

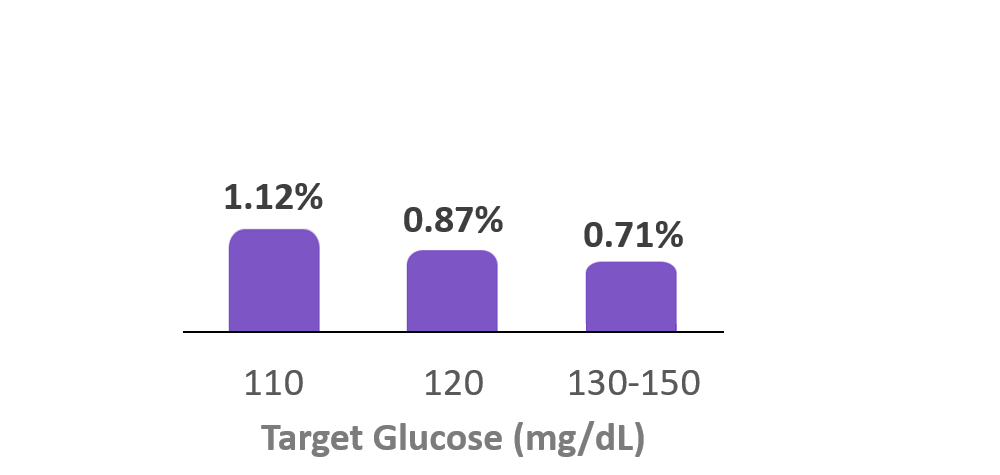
Time in Hypoglycemia
(<70 mg/dL)

Adult vs Pediatric Cohorts
The downloadable infographics below offer snapshot summaries of the results for the adult (age 18+) and pediatric (age 2-17) cohorts.
Why Real-World Evidence Matters
The Omnipod 5 pivotal trial found this automated insulin delivery (AID) system led to improved glycemic measures in the participants, reducing HbA1c by 0.38% in adults/adolescents and 0.71% in children.11 Real-world data provides an additional lens that can tell us whether the results researchers achieved in a more controlled setting of a study also bear out in everyday practice among a diverse population. In the case of Omnipod 5, we’re proud to see that it demonstrates pivotal trial outcomes, for time in and below range, under real world conditions.12
Why Selection Criteria Matters— Omnipod 5 Data is Uploaded Automatically
We know that datasets that rely on manual upload of pump data have selection bias and are likely to have skewed glycemic results that are not reflective of all clinical users.11 By contrast data from Omnipod 5 users is uploaded automatically, which minimizes selection bias.8 You can read the study summary and access the full study below.

Ready to Prescribe?
We offer not only a streamlined prescribing process through the pharmacy, but provider resources to help you support your patients.
1. Forlenza G, et al. Real-world evidence of Omnipod 5 Automated Insulin Delivery System use in 69,902 people with type 1 diabetes. Diabetes Technol Ther. 2024.
2. American Diabetes Association Professional Practice Committee; Summary of Revisions: Standards of Care in Diabetes—2024. Diabetes Care 1 January 2024; 47 (Supplement_1): S5–S10. https://doi.org/10.2337/dc24-SREV
3. Forlenza G, et al. Diabetes Technol Ther (2024). Real-world data from 69,902 individuals with type 1 diabetes using Omnipod 5 had a median TBR (<70 mg/dL) of 1.13%. Omnipod 5 results based on users with ≥90 days CGM data, ≥75% of days with ≥220 readings available.
4. Forlenza G, et al. Real-world evidence of Omnipod 5 Automated Insulin Delivery System use in 69,902 people with type 1 diabetes resulted in 93.7% time in Automated Mode. Diabetes Technol Ther. 2024.
5. Forlenza G, et al. Diabetes Technol Ther (2024). 13,342 out of 69,902 users with type 1 diabetes (19.1%) included in real-world analysis utilized MDI as prior therapy to the Omnipod 5 System. Omnipod 5 results based on users with ≥90 days CGM data, ≥75% of days with ≥220 readings available.
6. Forlenza G, et al. Diabetes Technol Ther (2024). Real-world data from 37,640 Omnipod 5 users with type 1 diabetes at the Target Glucose of 110 mg/dL had a median TIR (70-180 mg/dL) of 68.8%. Omnipod 5 results based on users with ≥90 days CGM data, ≥75% of days with ≥220 readings available.
7. Forlenza G, et al. Diabetes Technol Ther (2024). Real-world data from 69,902 individuals with type 1 diabetes using Omnipod 5 had a median TBR (<70 mg/dL) of 0.97%. Omnipod 5 results based on users with ≥90 days CGM data, ≥75% of days with ≥220 readings available.
8. Forlenza G, et al. Diabetes Technol Ther (2024). 28,612 adult Omnipod 5 users with type 1 diabetes utilizing the 110 mg/dL glucose target spent a median of 94.1% time in Automated Mode. Omnipod 5 results based on users with ≥90 days CGM data, ≥75% of days with ≥220 readings available. Device components including the Pod, CGM transmitter, and CGM sensor may be affected by strong radiation or magnetic fields. Device components must be removed (and the Pod and CGM sensor should be disposed of) before X-ray, Magnetic Resonance Imaging (MRI), or Computed Tomography (CT) scan (or any similar test or procedure). In addition, the Controller and smartphone should be placed outside of the procedure room. Exposure to X-ray, MRI, or CT, treatment can damage these components. Check with your healthcare provider on Pod removal guidelines.
9. Forlenza G, et al. Diabetes Technol Ther (2024). 6,525 Omnipod 5 users with type 1 diabetes at the Target Glucose of 110 mg/dL who utilized MDI as prior therapy had a time in range of 70.8%. Omnipod 5 results based on users with ≥90 days CGM data, ≥75% of days with ≥220 readings available.
10. Forlenza G, et al. Diabetes Technol Ther (2024). Data was uploaded automatically to the Insulet Cloud (via SIM card or wireless internet).
11. Brown S. et al. Diabetes Care. 2021;44:1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6–70 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time in range (70–180 mg/dL) as measured by CGM: ST vs Omnipod 5 use in adults/adolescents and children respectively (64.7% vs 73.9%, P<0.0001; 52.5% vs 68.0%, P<0.0001). Mean HbA1c: ST vs Omnipod 5 use in adults/adolescents and children respectively (7.16% vs 6.78%, P<0.0001; 7.67% vs 6.99%, P<0.0001). Median time in hypoglycemic range (<70 mg/dL) as measured by CGM: ST vs Omnipod 5 use in adults/adolescents and children respectively (2.0% vs 1.1%, P<0.0001; 1.4% vs 1.5%, P=0.8153). Median time in hypoglycemic range (<70 mg/dL; 12AM - < 6AM) as measured by CGM in adults/adolescents and children respectively: ST vs 3-mo Omnipod 5 (2.07% vs 0.82%, P<0.0001; 0.78% vs 0.78%, P=0.0456). Comparison is a relative change. Mean time 70-180 mg/dL (12AM-6AM) as measured by CGM in adults/adolescents and children, ST vs 3-mo Omnipod 5: 64.3% vs 78.1%, 55.3% vs 78.1%, P<0.0001, respectively.
Sherr JL, et al. Prospective trial in 80 participants with T1D aged 2 - 5.9 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time in range (70-180mg/dL) in very young children (2 - 5.9 yrs) as measured by CGM: ST = 57.2%, 3-mo Omnipod 5 = 68.1%, P<0.05.. Median time in hypoglycemic range (<70 mg/dL) as measured by CGM: ST vs. Omnipod 5 use in very young children (2 - 5.9 yrs) (3.43% vs. 2.46%, P=0.0204).
12. Forlenza G, et al. Diabetes Technol Ther (2024). 69,902 people with type 1 diabetes using the Omnipod 5 System at the 110 mg/dL Glucose Target had a TIR (70 mg/dL-180 mg/dL) of 67.8% and TBR (<70 mg/dL) of 1.62%. Omnipod 5 results based on users with ≥90 days CGM data, ≥75% of days with ≥220 readings available.
Brown S. et al. Diabetes Care. 2021;44:1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6 - 70 yrs [adults/adolescents (n= 128; aged 14-70 yrs) children (n=112; aged 6-13.9 yrs)]. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop phase. Mean time 70-180 mg/dL as measured by CGM in adults/adolescents and children, ST vs. 3-mo Omnipod 5: 64.7% vs. 73.9%; 52.5% vs. 68.0%, P<0.0001, respectively. Median time <70 mg/dL in adults/adolescents and children, ST vs. 3-mo Omnipod 5: 2.0% vs. 1.1%, P<0.0001; 1.4% vs. 1.5%, P=0.8153, respectively. Results measured by CGM.
Sherr JL, et al. Prospective trial in 80 participants with T1D aged 2 - 5.9 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time in range (70-180mg/dL) in very young children (2 - 5.9 yrs) as measured by CGM: ST = 57.2%, 3-mo Omnipod 5 = 68.1%, P<0.05.. Median time in hypoglycemic range (<70 mg/dL) as measured by CGM: ST vs. Omnipod 5 use in very young children (2 - 5.9 yrs) (3.43% vs. 2.46%, P=0.0204).
13. Wong et al. DTT. 2015; 17(8) 555-562
INS-OHS-02-2024-00150 v1.0

