
Reimagine insulin delivery for your patients living with T2D
Many healthcare providers are prescribing GLP-1s plus multiple daily injections (MDI) for their patients with type 2 diabetes (T2D), but research suggests that many people with T2D are not meeting the glycemic goals set by the American Diabetes Association.1 If they are struggling with MDI, there’s another way to manage your patients with T2D. It’s time to reduce the burden of managing diabetes—for them and for you.
Omnipod 5: Go Beyond GLP-1 Therapy in T2D
WATCH THE BEYOND THE BOLUS PODCAST RECORDING
A conversation between Davida Kruger, Certified Nurse Practitioner, and Leslie Barrett, Director of Medical Affairs at Insulet, recorded live at the American Diabetes Association's 85th Scientific Sessions.
Demonstrated clinical results
Omnipod 5 improved glycemic results for people with T2D diabetes who previously were on MDI2. Omnipod 5 is discreet and tubeless. It's the #1 requested pump by people with diabetes.3 It's simple to use so they can live more while thinking about their diabetes less.
SECURE-T2D Pivotal Trial data showed Omnipod 52:
Improved time in range by 20% — by nearly 5 hours per day
Lowered A1C by 2.1% from those with baseline A1C ≥9%
Reduced time in hyperglycemia by 37%
Demonstrated no increase in time in hypoglycemia
Reduced insulin use by 29%
In the 3-month SECURE-T2D study, 1 case of severe hypoglycemia was reported by an adult with type 2 diabetes during Omnipod 5 System use. The case was not related to Omnipod 5 System malfunction.
Learn from clinical investigators discussing the results of the SECURE-T2D Pivotal Trial
Panelists:

Trang Ly, MBBS, FRACP, PhD, Senior Vice President & Chief Medical Officer, Insulet

Kristin Castorino, DO, BC-ADM, Vice President of Clinical Research & Senior Investigator, Sansum Diabetes Research Institute

Davida Kruger, MSN, APN-BC, BC-ADM, Certified Nurse Practitioner, Henry Ford Health Division of Endocrinology, Diabetes, Bone and Mineral Disease

Viral Shah, MD, Adult Endocrinologist & Professor of Medicine, Division of Endocrinology & Metabolism, Indiana University Director of Diabetes Clinical Research IU Center for Diabetes & Metabolic Disease
Hear how Omnipod 5 simplifies T2D management for one endocrinologist’s patients
Dr. Luna, a board-certified endocrinologist, shares his perspective on what makes Omnipod 5 a smart choice for patients with T2D. He discusses the Omnipod 5 features that matter most to his patients and explains why he is proactive about using automated insulin delivery in his practice.
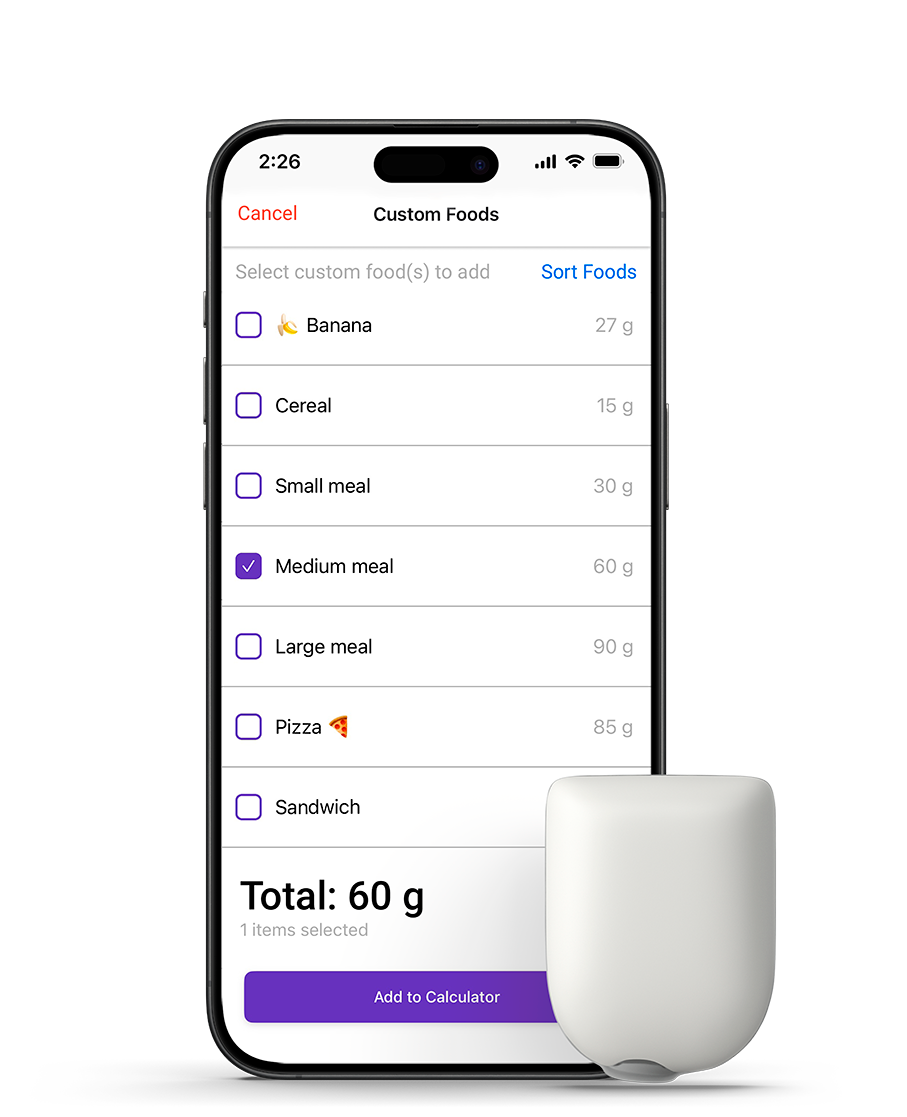

Empower patients to feel confident and in control
- Discreet, tubeless and waterproof*
- Simplified bolusing with the SmartBolus Calculator and the Custom Foods feature
- Automatic cannula insertion so you never see an injection needle
Omnipod 5 is compatible with leading sensor brands

Sensors are shown without necessary adhesive.
For a list of compatible smartphone devices, visit omnipod.com/compatibility.
Simple to access
Give your patients a simple way to try, start, and stay on
Omnipod 5.
Covered under Medicare Part D
Omnipod 5 is the only AID system covered under Medicare Part D without a C-peptide test
Simple pharmacy access
Patients can get Pods when they pick up their insulin
The majority of Omnipod 5 users with T2D pay less than $30 per month4
Patients with T2D are already achieving glucose goals with Omnipod 5
“With Omnipod 5, I have far fewer ups/downs of high and low sugars, and along with it, the terrible symptoms associated with that. Omnipod has been life changing for me.”
- Milli, Omnipod 5 user with T2D
Milli has an ongoing commercial relationship with Insulet.
Subscribe to stay in touch
Our newsletter for healthcare providers includes the latest Omnipod news and educational resources.
*The Omnipod 5 Automated Insulin Delivery System is indicated for use by individuals with type 1 diabetes mellitus in persons 2 years of age and older and type 2 diabetes mellitus in persons 18 years of age and older. The Omnipod 5 System is intended for single patient, home use and requires a prescription. The Omnipod 5 System is compatible with the following U-100 insulins: NovoLog®, Humalog®, and Admelog®. WARNING: SmartAdjust™ technology should NOT be used by anyone under the age of 2 years old. SmartAdjust™ technology should also NOT be used in people who require less than 5 units of insulin per day, as the safety of the technology has not been evaluated in this population.
1. Hankosky ER, Schapiro D, Gunn KB, Lubelczyk EB, Mitroi J, Nelson DR. Gaps Remain for Achieving HbA1c Targets for People with Type 1 or Type 2 Diabetes Using Insulin: Results from NHANES 2009-2020. Diabetes Ther. 2023;14(6):967-975. doi:10.1007/s13300-023-01399-0
2. Pasquel FJ, et al. JAMA Network Open (2025). Prospective pivotal trial in 305 participants with T2D aged 18-75 yrs. Study included a 14-day standard therapy (ST) phase followed by a 13-week Omnipod 5 hybrid closed-loop phase. In a subgroup analysis of 216 participants who used MDI as their prior insulin therapy mean HbA1c: baseline vs. 13-week Omnipod 5: 8.2% vs. 7.4%;. Mean time in range (70-180 mg/dL): ST vs. 13-week Omnipod 5: 45% vs. 66%, P<0.001. +4.8 hours/day. In a subgroup analysis of 68 participants with baseline A1c ≥ 9% Mean HbA1c: ST vs. 13-week Omnipod 5: 10.1% vs. 8.1%; (95% CI: -2.3%, -1.9%). Mean time <70 mg/dL as measured by CGM: ST = 0.2%, 3-mo Omnipod 5 = 0.2%, P<001. Mean time >180 mg/dL as measured by CGM: ST = 54%, 3-mo Omnipod 5 = 34%, P<0.001. Mean total daily dose (TDD): ST = 0.8U/kg/day, 3-mo Omnipod 5 = 0.57U/kg/day, P<0.001. Comparison is relative change.
3. Omnipod was the pump most frequently requested by people with T1D & T2D in a survey with Endocrinologists conducted by dQ&A across the United States; n=77 (T1) and n=63 (T2), H2 2024; P.25.
4. Source: IQVIA OPCL. Majority is defined over 70%. Based on paid claims for Omnipod 5 G6 Pods with a type 2 diabetes diagnosis code. Includes commercial and Medicare claims
© 2025 Insulet Corporation. Omnipod, the Omnipod 5 logo and SmartAdjust are trademarks or registered trademarks of Insulet Corporation in the United States of America and other various jurisdictions. All rights reserved. Dexcom, Dexcom G6, and Dexcom G7 are registered or unregistered trademarks of Dexcom, Inc. in the United States and/or other countries and are used with permission. The sensor housing, FreeStyle, Libre, and related brand marks are marks of Abbott and used with permission. All other trademarks are the property of their respective owners. The use of third party trademarks does not constitute an endorsement or imply a relationship or other affiliation.
INS-OHS-05-2025-00097 v2.0

