Omnipod® 5 Clinical Outcomes
Strong clinical results. Freedom from tubes.
We are committed to improving your patients’ clinical outcomes across type 1 and type 2 Diabetes, while providing them with the tubeless automated insulin delivery they love.
Demonstrated in the Real World
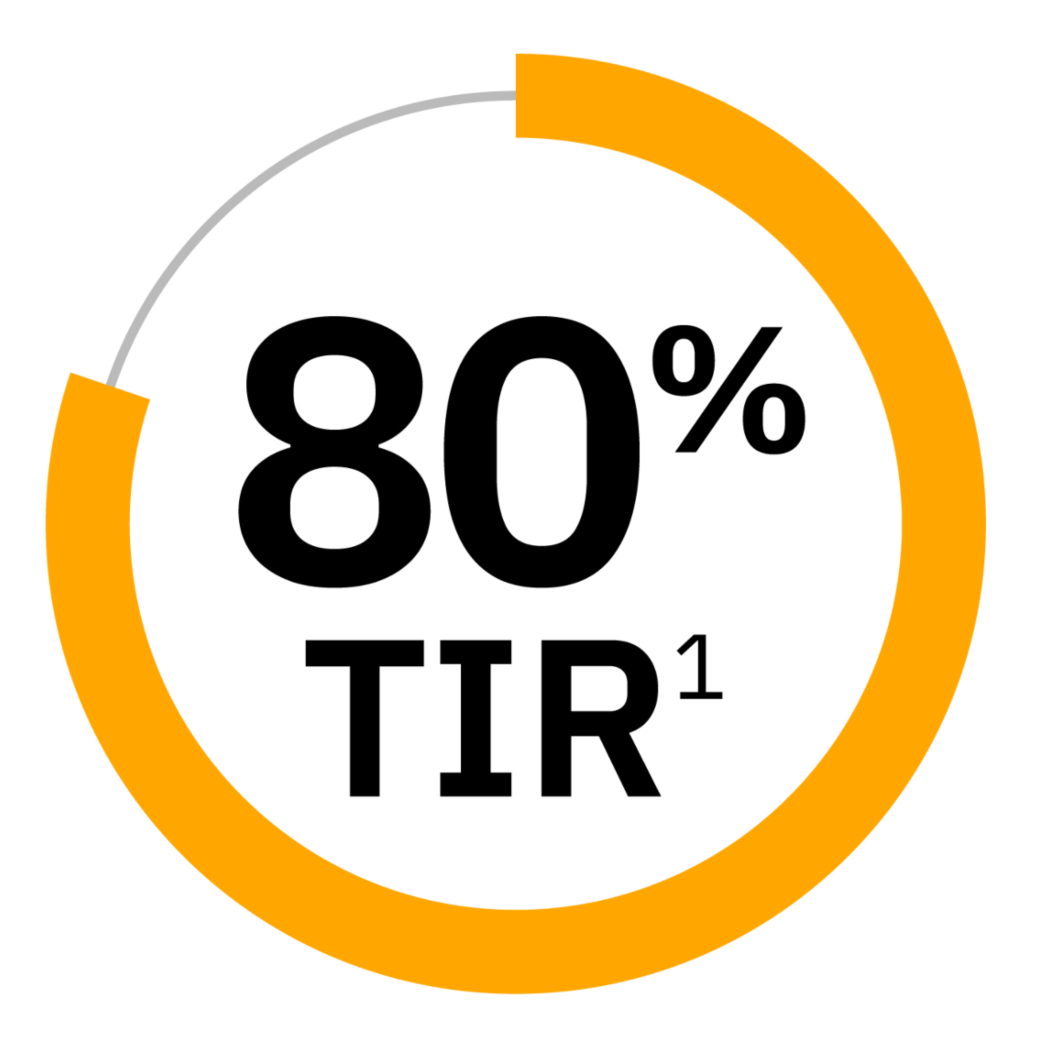
80% Time in Range1
Retrospective analysis of real-world data from 37,640 users with type 1 diabetes using Omnipod 5 who utilized the 6.1 mmol/L or 110 mg/dL glucose target of which 18.3% had 80% Time in Range (3.9-10.0 mmol/L or 70-180 mg/dL) or greater.
Read the full real world evidence publication


“Omnipod has helped my A1C tremendously. I get back time with my friends and family, and it’s made my job easier.”
Garrett V.
On air personality
Living with type 1 diabetes
Sponsored Omnipod 5 user
Podder® since 2023
Omnipod 5 RADIANT Randomized Controlled Trial2
The Omnipod 5 RADIANT trials is the first RCT to evaluate the direct transition from multiple daily injections to AID. Adults and children not meeting clinical targets showed
Adults with T1D Achieved:
Increased Time in Range by an average of 22%
(over 5 hours per day)
Lowered A1C by 0.8%
No increase in hypoglycemia
Children and Adolescents with T1D achieved:
Increased Time in Range by an average of 20%
Lowered A1C by 0.8%
No increase in hypoglycemia


"It helps me feel like a normal little kid, just with a little bit of help.”
Romey T.
Living with type 1 diabetes
Sponsored Omnipod 5 user
Podder® since 2019
Omnipod 5 SECURE-T2D Trial3
The SECURE-T2D trial, the first and most racially diverse type 2 diabetes pivotal study4, demonstrated improved clinical outcomes and quality of life in this population.
84% of participants were not carb-counting before enrollment
55% of participants were using a GLP-1
Adults with T2D achieved:
Lowered A1C by 2.1%
No increase in hypoglycemia
"I am a nurse and I know the importance of controlling my diabetes but unfortunately, I would miss my insulin injections…I was on a dangerous path. The ups and downs of high and low sugars are gone, and along with it, the terrible symptoms associated with that. Omnipod has been life changing for me.”
Milli
Nurse
Living with type 2 diabetes
Sponsored Omnipod 5 user
Podder® since 2023
1. Forlenza G, et al. Diabetes Technol Ther. 2024. 26(8):514-525. Retrospective analysis of real-world data from 37,640 users with type 1 diabetes using Omnipod 5 who utilized the 6.1 mmol/L or 110 mg/dL glucose target of which 18.3% had 80% Time in Range (3.9-10.0 mmol/L or 70-180 mg/dL) or greater. Omnipod 5 results based on users with ≥90 days CGM data, ≥75% of days with ≥220 readings available.
2. Wilmot E, et al. Presented at: ATTD; March 19-22, 2025; Amsterdam, NL. A 13-week randomized, parallel-group clinical trial conducted among 188 participants (age 4-70) with type 1 diabetes in France, Belgium, and the U.K., comparing the safety and effectiveness of the Omnipod 5 System versus multiple daily injections with CGM.
3. Pasquel FJ, et al. JAMA Network Open (2025). Prospective pivotal trial in 305 participants with T2D aged 18-75 yrs. Study included a 14-day standard therapy (ST) phase followed by a 13-week Omnipod 5 hybrid closed-loop phase. Mean time in range (70-180 mg/dL): ST vs. 13-week Omnipod 5: 45% vs. 66%, P<0.001. Mean HbA1c: ST vs. 13-week Omnipod 5: 8.2% vs. 7.4%, P<0.001. In a subgroup analysis of 68 participants with baseline A1c ≥ 9% Mean HbA1c: ST vs. 13-week Omnipod 5: 10.1% vs. 8.1%, P<0.001. Mean T2-DDAS total intensity score: ST = 2.5, 3-month Omnipod 5 = 2.2, P<0.001. Mean Proportion with T2-DDAS ≥ 2.0, no. (%): ST = 66%; 3-month Omnipod 5 = 55%, P<0.001
4. Based on published clinical trials in the current AID T2D landscape