Going Beyond GLP-1 Therapy in T2D with Omnipod® 5


Note: Davida Kruger has a commercial relationship with Insulet. Her views and opinions are her own.
It was such a pleasure presenting alongside Davida Kruger, MSN, APN-BC, BC-ADM at the American Diabetes Association (ADA) 85th Scientific Sessions in June. Thank you to those who attended our Diabetes Learning Byte presentation in person. For those of you who could not make it, we wanted to highlight some of the most significant discussion points and learnings from the important conversation we had, and the key insights we shared.
GLP-1s have demonstrated substantial clinical benefits for many people with type 2 diabetes (T2D), but despite their growing use, many of these people have A1C levels that are greater than the <7% glycemic goal recommended by the ADA.1 Only 25.2% of people with T2D using mealtime insulin achieve this target, and the mean A1C for this population is 8.6%. This puts them at greater risk of microvascular and macrovascular complications.2
Why are these individuals struggling? Because multiple daily injections (MDI) are challenging. In her practice, Davida hears about these struggles all the time. During the presentation, she recounted some common themes. Her patients tell her:
“I’m scared of lows.”
“I don’t want to give an injection in public.”
“I forget to take my insulin.”
“I find carb counting challenging.”
“MDI takes so much work.”
Davida encouraged us to think of these admissions not as complaints but as cues that patients need an alternative therapy. In her words, “For too long, the struggle has been attributed to the failings of the patient, when we should have been looking at the shortcomings of the therapy. They may not be aware of the benefits and simplicity that automated insulin delivery (AID) offers. It is up to us to introduce it as an option that pairs well with their GLP-1.”
There is a perception that patients with certain characteristics will struggle to adapt to AID. AID systems are thought of as too complex. Patients may not be proficient at counting carbs. Even the fact that they have been struggling on MDI may be interpreted to suggest that they would also struggle on AID. Too often this means they are not offered the option to switch.
Our SECURE-T2D study, which we are proud to say was the first and most racially diverse study of AID in T2D published to date, reflects the U.S. population living with T2D.3 Participants were largely naïve to technology, on high doses of insulin, not carb counting, or already on advanced therapies like GLP-1s. These are individuals who traditionally have not been considered as candidates for AID.
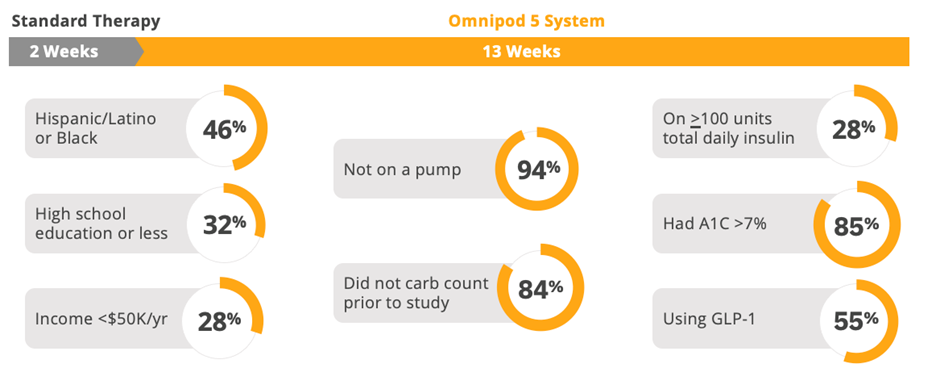

SECURE-T2D: Pivotal study demographics reflect U.S. population3
So, let’s see if those assumptions about this patient population were borne out in the clinical outcomes.
Findings from the SECURE-T2D study make clear that people living with T2D can benefit from Omnipod 5—regardless of their lack of experience using pumps, regardless of if they were carb counting prior to the study, regardless of how much insulin they use, and even if they were already using a GLP-1.4
Participants lowered their A1C by 0.8% in the full population, but for those with a starting baseline of ≥9%, A1C reduced by 2.1%. Time in range (TIR) improved by nearly 5 hours a day (70-180 mg/dL). And all this was achieved with no increase in time in hypoglycemia.*5 As Davida said at ADA, not only are these patients good candidates for pumps, arguably they are the patients who can benefit the most from them.
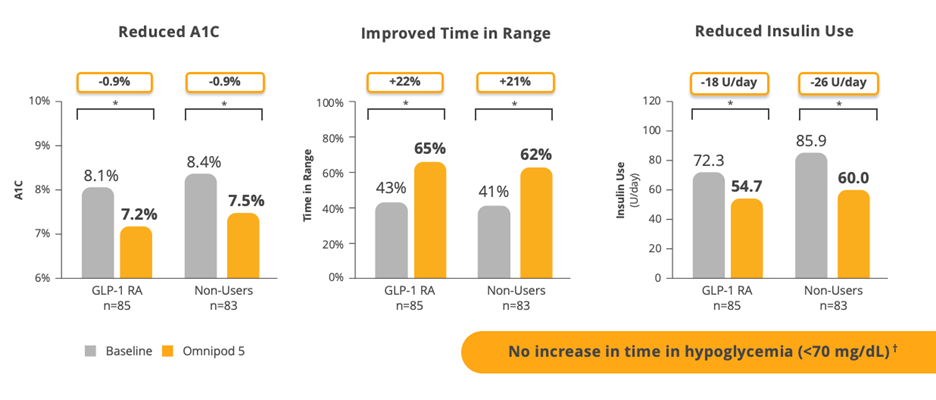

Data shown as mean *P<0.001. Interaction P-value comparing change in outcome across subgroups was P>0.05.
†In the 3-month SECURE-T2D study, one case of severe hypoglycemia was reported by an adult with type 2 diabetes during Omnipod 5 System use. The case was not related to Omnipod 5 System malfunction.
Just over half of the study participants were on a stable dose of GLP-1 prior to enrolling in the study, but most of them were not achieving glycemic targets at baseline. When looking at outcomes demonstrating improvements in A1C and TIR, it really doesn’t matter whether they were on a GLP-1 or not. Omnipod 5 provided consistent, significant improvements in glycemic metrics across the board. This suggests that adults with T2D who are taking GLP-1s and require insulin therapy can still benefit from AID.
Now, let’s think back to those cues that Davida mentioned. Not only were they signals that MDI may not be working out for those patients, but they were also evidence of diabetes-related distress. These patients were afraid, embarrassed, and overwhelmed. Diabetes distress is real, and managing this disease can take a huge emotional and mental toll on individuals.
In the SECURE-T2D study, people living with T2D reported a clinically meaningful reduction in diabetes distress.6 Participants endorsed Omnipod 5: 90% said they would recommend Omnipod 5 to a family member or friend, 91% felt Omnipod 5 made it easy to deliver mealtime insulin, and nearly 80% said they wanted to continue using Omnipod 5 after the study ended.7-9 For people living with T2D, Omnipod 5 not only represents improved clinical results, it also means their insulin management does not have to be as burdensome.
When we received this data around diabetes distress, we were not surprised by the findings. Our mission at Insulet is to simplify life with diabetes, and through multiple innovations we’ve stood firm to that. Omnipod 5 is simple and intuitive. It is tubeless, waterproof,** and discreet. It is compatible with leading sensor brands and offers personalized therapy with the option to use a Controller or compatible smartphone.*** We’ve recently launched the Custom Foods feature, which allows programming of small, medium, and large meals, or breakfast, lunch, and dinner, to simplify mealtime bolusing. Omnipod 5 is accessible. Cost is often a perceived barrier to introducing AID, but Omnipod 5 is covered under Medicare part D, with no C-peptide requirements, and most of our users pay less than $30 dollars per month.
Our clinical data shows how people living with T2D can adopt easy-to-use technology and improve clinical outcomes and alleviate diabetes distress. Omnipod 5 is a beautiful complement to existing GLP-1 therapy—especially for those struggling with MDI. We now have the opportunity to bring this unique therapy to the underserved T2D population.
If you have patients who tell you that they fear lows, that they don’t want to give an injection in public, or that MDI is hard, recognize these as the cues they are. They aren’t making excuses. They are trying to ask for an easier way to manage their diabetes, and you have an opportunity to make a difference for them with Omnipod 5.