TECHNOLOGY MADE SIMPLE FOR YOU



OMNIPOD® 5
IS SMALL BUT ITS
IMPACT IS
HUGE


Automated insulin delivery helps protect against highs and lows, day and night1,2
No multiple daily injections, insulin pump tubing or finger pricks‡
Share the load with a system that’s always adjusting, so you don’t have to*
Suitable for people with type 1 diabetes ages 2 years and older
Integrates with the Dexcom G6 sensor and the FreeStyle Libre 2 Plus sensor. A separate prescription is required for the sensor.
The Dexcom G6 and FreeStyle Libre 2 Plus sensors are sold separately.
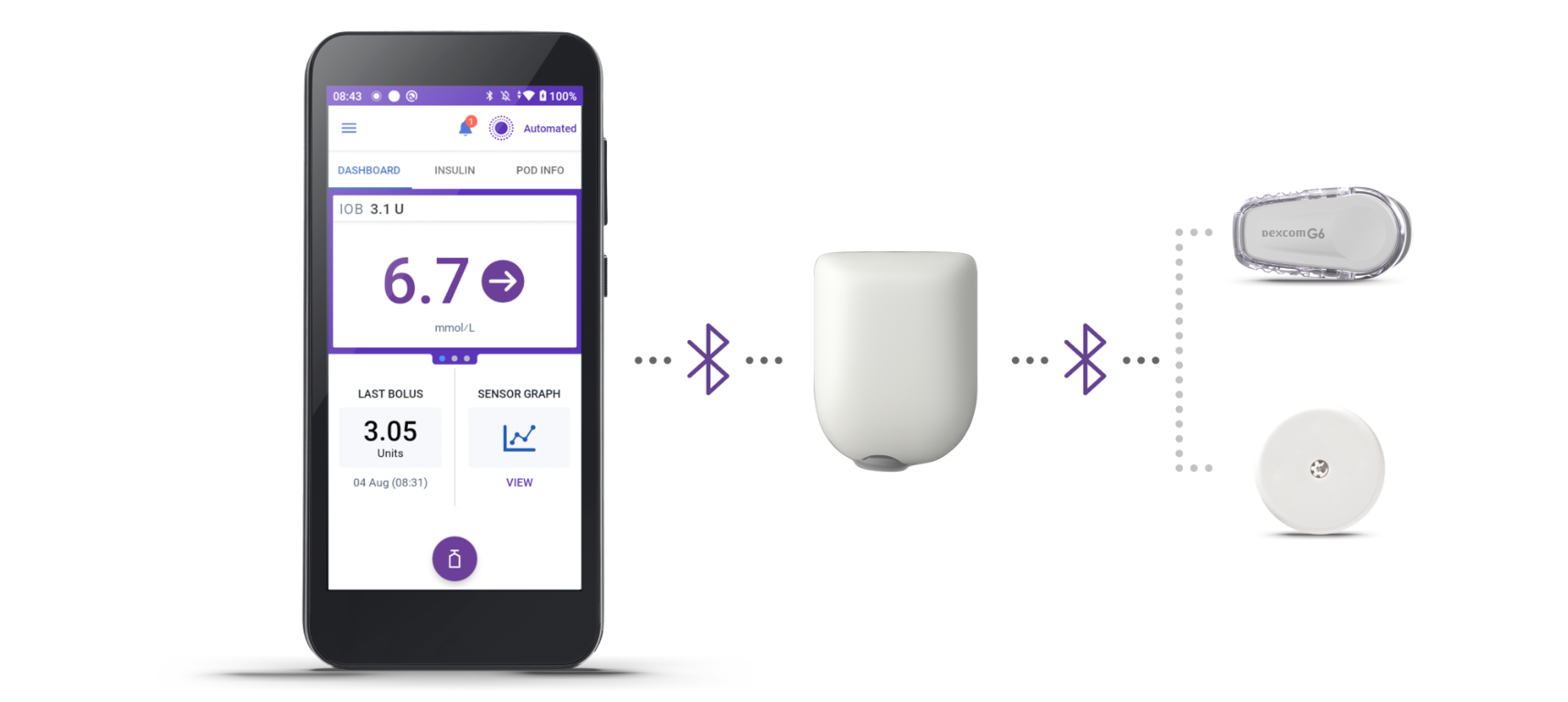
How it works
3 simple parts. One continuous loop of communication
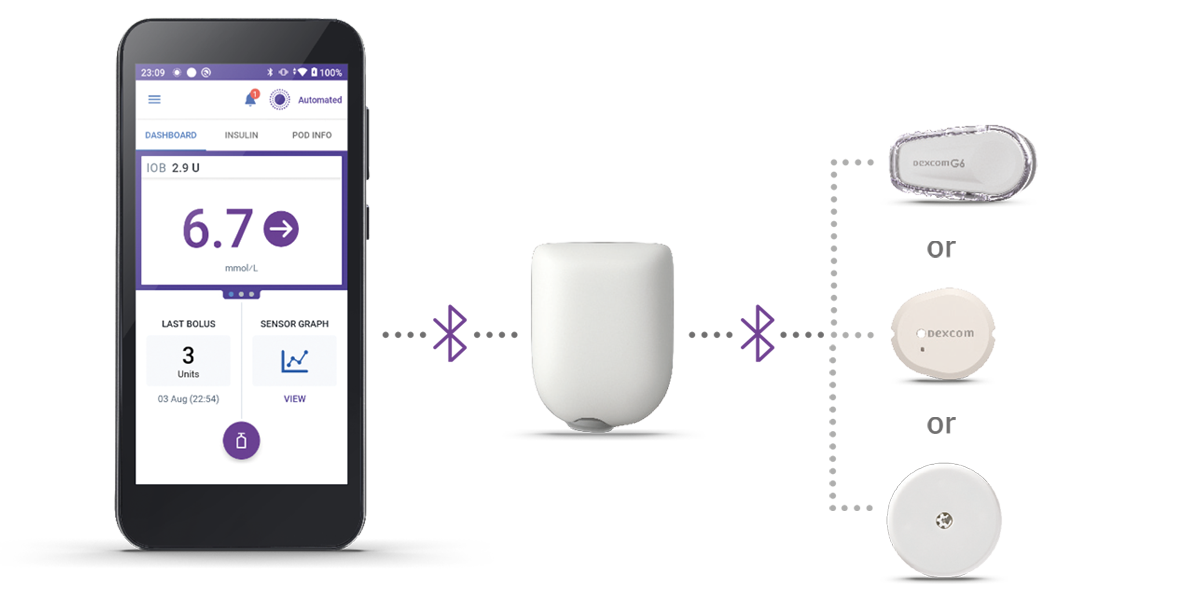

Omnipod 5 Controller
Take control of the system with the Omnipod 5 Controller. Monitor and control the Pod's operations using Bluetooth® wireless technology.
Pod
Tubeless, wearable, and waterproof†, the Pod, with built-in SmartAdjust™ technology, sits on the body and automatically adjusts insulin delivery for up to 3 days or 72 hours.
Sensor
Continuously sends glucose values to the Pod, so you can get real-time data without the finger pricks‡.
When paired with an compatible Continuous Glucose Monitor (CGM) sensor, Omnipod 5 can give constant and automatic insulin delivery to help protect from highs and lows, day and night1,2. Omnipod 5 is compatible with Dexcom G6 and Freestyle Libre 2 Plus Sensor.
Here’s what our Podders have to say about Omnipod®:
Omnipod 5 has allowed me to get a good night sleep. That's the first time I can say that in a long time.
Alvin
Podder since 2017
It helps me feel like a normal kid, just with a little bit of help.
Romey T.
Sponsored Omnipod® User
& Podder since 2019
I don’t have to spend as much time thinking about diabetes.
Clare F.
Podder® since 2013
Try it for yourself
Nothing beats firsthand experience. The free Pod Experience Kit* (PEK) contains a needle-free nonfunctioning sample Pod for you to wear. It also includes a quick-start guide providing you with more information about Omnipod.
Talk to an Omnipod® Specialist
Still have more questions about the Omnipod® System? Enter your information below and one of our Omnipod® Specialists will call you in 24-48 hours for a one-to-one chat. Or you can call us direct at 0800 011 6132* (option 4) or +44 20 3887 1709 (option 4) if calling from abroad.
Important: Please note that the customer must be residing in the United Kingdom to be eligible for the Omnipod 5 Automated Insulin Delivery System.
The Pod Experience Kit contains a needle-free Pod that does not deliver insulin. Controller is not included.
Requires Dexcom G6 or FreeStyle Libre 2 Plus sensor. Bolus for meals and corrections are still needed.
†The Pod has an IP28 rating for up to 7.6 metres (25 feet) for 60 minutes. The Omnipod 5 Controller is not waterproof. Please refer to sensor manufacturer user guide for sensor waterproof rating.
‡ Finger pricks required for diabetes treatment decisions if symptoms or expectations do not match readings.
§ The Dexcom G6 sensor must be used with the Dexcom G6 App. The Dexcom G6 receiver is not compatible with Omnipod 5. The FreeStyle Libre 2 Plus sensor cannot be used with the LibreLink App, LibreView App and LibreLinkUp App, and it must be started with the Omnipod 5 App only.
1. Brown S. et al. Diabetes Care. 2021;44:1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6 - 70 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closedloop (HCL) phase. Mean time >10.0 mmol/L or >180mg/dL (12AM-<6AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 32.1% vs. 20.7%; 42.2% vs 20.7%, P<0.0001, respectively. Mean time >10.0 mmol/L or >180mg/dL (6AM-<12AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 32.6% vs. 26.1%; 46.4% vs 33.4%, P<0.0001, respectively. Mean time <3.9 mmol/L or <70 mg/dL (12AM-<6AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 3.64% vs. 1.17%, P<0.0001; 2.51% vs. 1.78, P=0.0456, respectively. Mean time <3.9 mmol/L or <70 mg/dL (6AM-<12AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 2.64% vs. 1.37%, P<0.0001; 2.13% vs. 1.98%, P=0.2545, respectively.
2. Sherr J. et al. Diabetes Care. 2022; 45:1907-1910. Single-arm multicenter clinical trial in 80 pre-school children (aged 2-5.9 yrs) with T1D. Study included a 14-day standard therapy (ST) phase followed by a 3-month AID phase with Omnipod 5 system. Mean time >10.0 mmol/L or >180mg/dL (12AM-<6AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 38.4% vs. 16.9%, P<0.0001, respectively. Mean time >10.0 mmol/L or >180mg/dL (6AM-<12AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 39.7% vs. 33.7%, P<0.0001, respectively. Mean time <3.9 mmol/L or <70 mg/dL (12AM-<6AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 3.41% vs. 2.13%, P=0.0185. Mean time <3.9 mmol/L or <70 mg/dL (6AM-<12AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 3.44% vs. 2.57%, P=0.0799.