Say Hello to Omnipod® 5

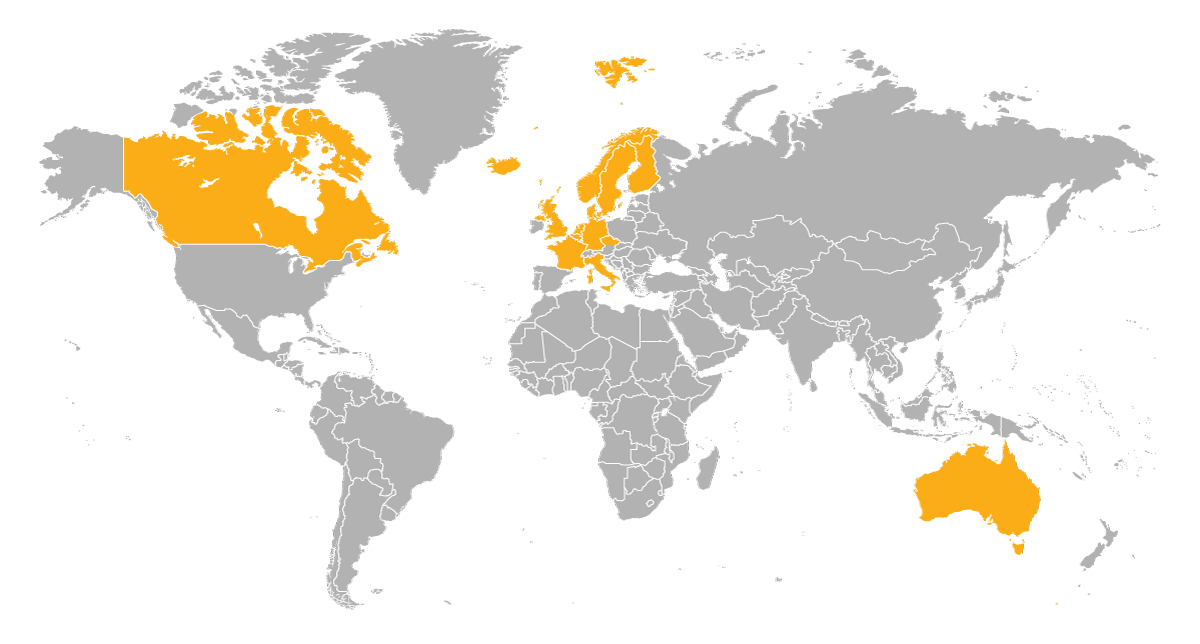
Omnipod 5 is now available in 14 markets: Australia, Belgium, Canada, Denmark, Finland, France, Germany, Italy, Netherlands, Norway, Sweden, Switzerland, the U.K., and the U.S.
Managing type 1 diabetes just got easier for your patients. They can rely on the system that makes automated§ basal insulin delivery adjustments every 5 minutes—so they don’t have to.

Omnipod® 5 Automated Insulin Delivery
The first wearable, tubeless, hybrid closed loop system that integrates with the leading sensor brands∞, for your patients with type 1 diabetes (T1D) aged 2 years and older.
∞Compatible with Dexcom G6, Dexcom G7 and FreeStyle Libre 2 Plus sensors
Innovation at its best with three simple parts!
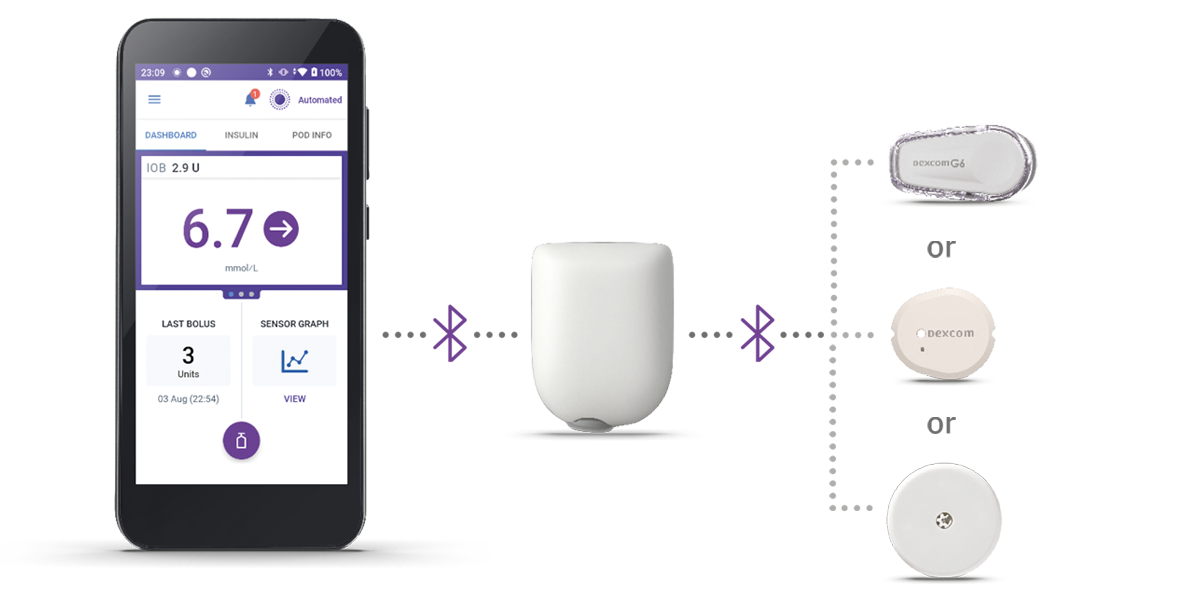

Omnipod 5 is compatible with select sensors, so people with diabetes can experience the freedom of automated insulin delivery and improved time in range!1,2
Omnipod 5 in clinicial trials:1,2
Increased
time in range
Reduced
HbA1c
Helped protect against highs and lows
All this without multiple daily injections, insulin pump tubing, and routine finger-stick testing.†
Training at your fingertips
Whether you’re new to Omnipod or ready to deepen your knowledge, our expert-led educational webinars and Within Range: Demystifying Diabetes Tech podcast provide practical guidance on prescribing and supporting Omnipod 5 in clinical practice.
Hear experiences from some of the first Omnipod® 5 Podders
Stay in the loop
Sign up for updates on product enhancements and training and education sessions in your country.
† Bolus required for meals and corrections. If your glucose alerts and readings from the sensor do not match symptoms or expectations, use a blood glucose meter to make diabetes treatment decisions.
§Compatible with the Dexcom G6, Dexcom G7 and FreeStyle Libre 2 Plus Sensors. Sensor is required for Automated Mode. Boluses for meals and corrections are still necessary. Sensors are sold separately and require a separate prescription. The Dexcom Sensors must be used with the respective Dexcom mobile app. The Dexcom receiver is not compatible. Devices compatible with Dexcom apps can be found at https://www.dexcom.com/compatibility.
1. Brown SA, et al. Diabetes Care. 2021;44:1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6 - 70 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time in range (3.9-10.0 mmol/L or 70-180 mg/dL) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5 = 64.7% vs. 73.9%, 52.5% vs. 68.0%, respectively (p<0.0001). Mean HbA1c: ST vs. Omnipod 5 use in adults/adolescents (14-70 yrs) and children (6-13.9 yrs), respectively (7.16% vs. 6.78% or 55 mmol/mol vs. 51 mmol/mol, p<0.0001; 7.67% vs. 6.99%or 60 mmol/mol vs. 53 mmol/mol), p<0.0001). Mean time in hyperglycaemic range (>10.0 mmol/L or >180 mg/dL as measured by CGM) in adults/adolescents and children ST vs. 3-mo Omnipod 5: 28.9% vs. 22.8%; 44.8% vs. 29.7%, p<0.0001, respectively. Mean time in hypoglycaemic range (<3.9 mmol/L or <70 mg/dL as measured by CGM) in adults/adolescents and children ST vs. 3-mo Omnipod 5 = 1.89% vs. 1.32% (p<0.0001) and 2.21%, vs. .78% (p<0.0456), respectively. In a 3-month clinical study, 3 case of severe hypoglycaemia and 1 case of diabetic ketoacidosis (DKA) were reported during Omnipod 5 System use. These cases were not related to automated insulin delivery malfunction.
2. Sherr JL, et al. Diabetes Care. 2022; 45:1907-1910. Single-arm multicenter clinical trial in 80 pre-school children (aged 2-5.9 yrs) with T1D. Study included a 14-day standard therapy (ST) phase followed by a 3-month AID phase with Omnipod 5 system. Mean HbA1c as measured in very young children, ST vs. Omnipod 5 use: 7.4% vs. 6.9% or 57 mmol/mol vs. 53 mmol/mol; (p<0.0001). Mean time in range (3.9-10.0 mmol/L or 70-180 mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 57.2% vs. 68.1%, p<0.0001. Mean time in hyperglycaemic range (>10.0 mmol/L or >180 mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 39.4% vs. 29.5%, p<0.0001, respectively. Mean time in hypoglycaemic range (<3.9 mmol/L or <70 mg/dL as measured by CGM) ST = 3.43% vs. Omnipod 5: 2.46%, p<0.0001.
The Omnipod 5 Automated Insulin Delivery System is a single hormone insulin delivery system intended to deliver U-100 insulin subcutaneously for the management of type 1 diabetes in persons aged 2 and older requiring insulin. The Omnipod 5 System is intended for single patient use. The Omnipod 5 System is indicated for use with NovoLog®/NovoRapid®, Humalog®, Trurapi®/Truvelog®/Insulin aspart Sanofi®, Kirsty®, and Admelog/Insulin lispro Sanofi U-100 insulin. Refer to the Omnipod® 5 Automated Insulin Delivery System User Guide and www.omnipod.com/safety for complete safety information including indications, contraindications, warnings, cautions, and instructions.