Why Omnipod® 5?

Previously unmet needs
Understand the challenges of managing T1D with traditional solutions like multiple daily injections (MDI).
Current landscape of T1D management
of adults in England are not meeting the HbA1c target of 58 mmol/mol (7.5%).1
Children and young people in England and Wales have an average HbA1c of 60 mmol/mol—above NICE’s recommended target of 48 mmol/mol (6.5%).2
Elevated levels of HbA1c increased risk of future complications.3
Most people with T1D do not meet their glycaemic targets.4
Children and young people using MDI had a higher average HbA1c than those using AID systems; 73.0 mmol/mol (8.8%) vs. 59.1 mmol/mol (7.6%), respectively.2,*
Burden of MDI
57% of people with diabetes in a US survey reported skipping insulin injections they knew they should take.5
Continuously managing blood glucose is a substantial mental burden for people with T1D and their families or carers.6
Consensus guidelines recommend AID systems
An overview of UK automated insulin delivery (AID) guidelines and NICE recommendations for AID (hybrid closed loop) systems.
NICE Guidelines for AID system adoption
Push for adoption
NICE has issued final guidance recommending the use of AID systems.6
Clinical evidence
NICE recognises the clinical evidence that suggests AID systems are more effective than standard care in achieving glycaemic control.6
5-year rollout
NICE and NHS England plan a five-year phased rollout is underway, prioritising children, young people, pregnant women, and current insulin pump users.6
“HCL [hybrid closed loop] systems are recommended as an option for managing blood glucose levels in type 1 diabetes for children and young people.”
- NICE TA Guidance6
Understand how NICE recommendations are advocating for AID systems
ADA and EASD 2021 report7
“Hybrid closed loop systems are the most effective means of maintaining glucose in the normal range in people with type 1 diabetes.”
Endocrine Society’s AID consensus report8
Strongly consider recommending AID systems to all people with T1D to improve glycaemic control:
School-aged children (7-14 years)
Adolescents/adults
Why AID is a game changer
Improved glycaemic results
Compared to non-AID pump and sensor, AID systems significantly increased time-in-range and significantly reduced time-below-range.9
Simplified T1D management
AID systems, compared to MDI, make it easier to manage diabetes so more people can reach their glycaemic goals.10
Patient confidence
Adult users and parents of children felt more confident in staying safe from the risk of hypoglycaemia compared to prior therapy.11,12
Improved QoL
Parents of children reported improved quality of life and mental well‑being compared to prior therapy.11
Watch leading clinicians discuss AID system features and benefits
Omnipod 5 simplifies AID technology for all patient types
A closer look at Omnipod 5’s indications, features, and components to understand its functionality and ease of use.


Who is Omnipod 5 for?
The Omnipod 5 AID System is the first wearable, tubeless, hybrid closed loop system for people with T1D aged 2 years and older requiring insulin.
Key benefits of Omnipod 5
Proven to improve clinical outcomes including time in range (TIR) versus MDI for both adults and children13
Integrates seamlessly with the leading sensor brands
A tubeless and waterproof* AID system to further simplify life with diabetes
Omnipod 5 works with 3 simple parts
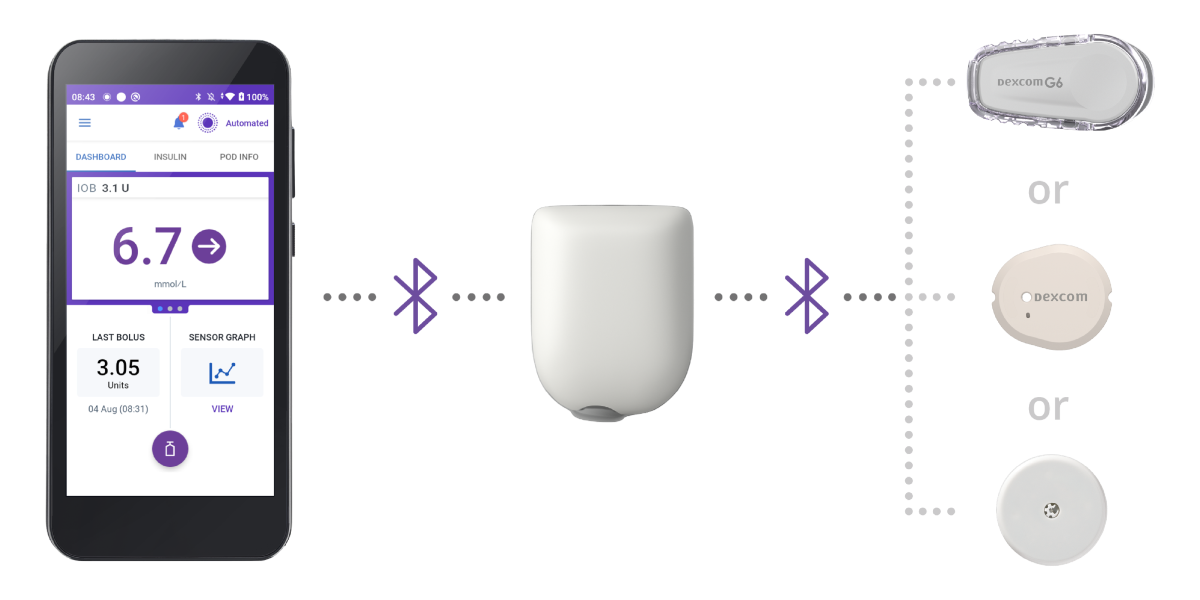

Controller
The Omnipod 5 Controller allows the patient to monitor and control the Pod.
Pod
Tubeless, wearable, and waterproof*, the Pod sits right on your patient’s body and automatically† adjusts insulin delivery every 5 minutes for up to 3 days (72 hours).
Sensor
Continuously sends glucose values to the Pod, so your patients can get real-time data without fingerpricks.‡
Learn more about the Omnipod 5 system
Omnipod 5’s SmartAdjust™ algorithm automatically manages insulin delivery
SmartAdjust™ Technology automatically increases, decreases, or pauses basal insulin delivery — adjusting every five minutes to the patient’s needs, helping to correct highs and protect from lows.14,15
†Automated Mode requires compatible CGM Sensor.
‡Fingerpricks required for diabetes treatment decisions if symptoms or expectations do not match readings.
Omnipod 5 is proven to improve clinical outcomes
Review the latest clinical studies, real-world data, and patient-HCP experiences demonstrating Omnipod 5’s effectiveness and safety.
Clinical studies
Omnipod 5 improves glycaemic control
Key Findings14,15,*,†:
- Significantly improved HbA1c vs. baseline
- Significantly improved TIR vs. standard therapy
Omnipod 5 improves quality of life
Key Findings11,12,‡:
- Reduced diabetes distress
- Increased confidence in staying safe from the risk of hypoglycaemia
- 38% improvement in caregiver sleep quality
*Omnipod 5 significantly improved HbA1c and TIR in users from 2 years of age in pivotal trials. Omnipod 5 reduced HbA1c by 0.5% or 6 mmol/mol in very young children and 53% of children and 66% of adults/adolescents had an HbA1c of less than 7% or 53 mmol/mol. Required a Dexcom G6 sensor. Results are compared to baseline (MDI or continuous subcutaneous insulin infusion (CSII), HbA1c only) or standard therapy (MDI or CSII, other outcomes).14,15
†The Omnipod 5 pivotal trials were multicentre, single-arm outpatient studies in the US. A total of 321 participants were enrolled: very young children aged 2–5.9 years, diagnosed with T1D for any duration and participants aged 6–70 years, diagnosed with T1D for ≥6 months. Additionally, patients were included if they had HbA1c <10.0% or 86mmol/mol at screening visit. Prior insulin therapy with either CSII or MDI was permitted, with no minimum requirement for total daily dose of insulin. There were no restrictions on eating or exercise throughout the study period.14,15
‡Adults reported reduced diabetes distress compared to prior therapy—mean diabetes distress scale (T1-DDS) score of 1.48 vs. 1.64. Adults reported lower stress when eating compared to prior therapy—eating distress survey (T1-DDS Eating Distress Subscale) score of 1.74 vs. 1.97. Parents of children reported improved quality of life and mental well-being compared to prior therapy. Adult users and parents of children felt confident in staying safe from the risk of hypoglycaemia compared to prior therapy—mean hypoglycaemia confidence survey (HCS) score of 3.59 vs. 3.34 and 3.65 vs. 3.52, respectively. Parents of children reported a 38% improvement in sleep quality compared to prior therapy—mean PSQI Overall Sleep Quality Subscore of 0.70 vs. 1.13, (comparison is relative change).11,12
Omnipod 5 is changing real-world diabetes management
Learn more and optimise your approach to Omnipod 5 with practical tips from diabetes experts.
Hear directly from Omnipod 5 Podders
Lydia
Lea
Timothie
Consistent support for you and your practice
Insulet is commited to simplifying diabetes care, sustainability efforts, and providing you with the resources to best onboard and support your patients.
Insulet’s mission
Our mission is to improve the lives of people with diabetes and enable people with diabetes to enjoy simplicity, freedom, and healthier lives through innovative technology.
Training and Onboarding
Simulator App
Download the Simulator App for an interactive simulation and system overview of the Omnipod 5 AID System.
Expert-led webinar
Get expert insights on AID training, virtual education data, and how to streamline your clinic with Omnipod 5.
Book a meeting
Want to book product training or speak with your local Omnipod Specialist?
Learn how to get your patients started
Explore practical tools, training, and prescribing steps for Omnipod 5
References:
1. National Diabetes Audit. National Diabetes Audit 2020-21: Care Processes and Treatment Targets. NHS Digital; 2022. Accessed June 4, 2025. https://www.hqip.org.uk/wp-content/uploads/2022/07/REF243_NDA-CORE-REP1-CPTT_FINAL-v20220711.pdf
2. Royal College of Paediatrics and Child Health. National Paediatric Diabetes Audit (NPDA) annual reports. RCPCH; 2025. Accessed July 3, 2025. http://www.rcpch.ac.uk/resources/npda-annual-reports
3. DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391(10138):2449-2462. doi:10.1016/S0140-6736(18)31320-5
4. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016-2018. Diabetes Technology & Therapeutics. 2019;21(2):66-72. doi:10.1089/dia.2018.0384
5. Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) study. Diabetes Care. 2010;33(2):240-245. doi:10.2337/dc09-0918
6. National Institute for Health and Care Excellence (NICE). Hybrid Closed Loop Systems for Managing Blood Glucose Levels in Type 1 Diabetes (Technology Appraisal TA943). December 19, 2023. Accessed June 4, 2025. https://www.nice.org.uk/guidance/ta943
7. Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults: a consensus report by the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2021;64(12):2609-2652. doi:10.1007/s00125-021-05568-3
8. Phillip M, Nimri R, Bergenstal RM, et al. Consensus recommendations for the use of automated insulin delivery technologies in clinical practice. Endocr Rev. 2023;44(2):254-280. doi:10.1210/endrev/bnac022
9. Renard E, Forlenza GP, Brown SA, et al. Efficacy and safety of the automated Omnipod 5 system compared with insulin pump therapy in adults with type 1 diabetes: a randomized, controlled trial. Paper presented at: 17th International Conference on Advanced Technologies & Treatments for Diabetes (ATTD 2024); March 6-9, 2024; Florence, Italy.
10. Forlenza GP, DeSalvo DJ, Aleppo G, et al. Real-World Evidence of Omnipod® 5 Automated Insulin Delivery System Use in 69,902 People with Type 1 Diabetes. Diabetes Technol Ther. 2024;26(8):514-525. doi:10.1089/dia.2023.0578
11. Hood KK, et al. Diabetes Obes Metab. 2024;26(12):5569-5579. Single-arm, multicentre prospective study in 125 paediatric participants with T1D (83 children 6–11.9 yrs; 42 adolescents 12–17.9 yrs). All used Omnipod 5 for 3 months; validated age- and role-specific questionnaires (PAID, HCS, WHO-5, PSQI, IDSS, SUS) were completed by participants and caregivers at baseline and study end to evaluate psychosocial outcomes. Parents of children reported improved quality of life and mental well-being compared to prior therapy. Parents of children felt confident in staying safe from the risk of hypoglycaemia compared to prior therapy—mean hypoglycaemia confidence survey (HCS) score of 3.65 vs. 3.52. Parents of children reported a 38% improvement in sleep quality compared to prior therapy—mean PSQI Overall Sleep Quality Subscore of 0.70 vs. 1.13, (comparison is relative change).
12. Polonsky WH, et al. Diabetes Research and Clinical Practice. 2022;190:109998. Single-arm, multicentre prospective study in 115 adults with T1D (18–70 yrs). All used Omnipod 5 for 3 months; psychosocial questionnaires (T1-DDS, HCS, WHO-5, PSQI, IDSS, DTSQ, SUS) were completed at baseline and study end. Adults reported reduced diabetes distress compared to prior therapy—mean diabetes distress scale (T1-DDS) score of 1.48 vs. 1.64. Adults reported lower stress when eating compared to prior therapy—eating distress survey (T1-DDS Eating Distress Subscale) score of 1.74 vs. 1.97. Adult users felt confident in staying safe from the risk of hypoglycaemia compared to prior therapy—mean hypoglycaemia confidence survey (HCS) score of 3.59 vs. 3.34.
13. Wilmot E, et al. Presented at: ATTD; March 19-22, 2025; Amsterdam, NL. A 13-week randomized, parallel-group clinical trial conducted among 188 participants (age 4-70) with type 1 diabetes in France, Belgium, and the U.K., comparing the safety and effectiveness of the Omnipod 5 System versus multiple daily injections with CGM. Data on File RF-042025-00015.
14. Brown SA, et al. Diabetes Care. 2021;44(7):1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6-70 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time in range (3.9-10.0 mmol/L) in adults/adolescents as measured by CGM: ST = 64.7%, 3-mo Omnipod 5 = 73.9%, p<0.0001. Mean time in range (3.9-10.0 mmol/L) in children as measured by CGM: ST = 52.5%, 3-mo Omnipod 5 = 68.0%, p<0.0001. Mean HbA1c: ST vs. Omnipod 5 use in adults/adolescents (14-70 yrs) and children (6-13.9 yrs), respectively (7.16% vs. 6.78% or 55 mmol/mol vs. 51 mmol/mol, p<0.0001; 7.67% vs. 6.99% or 60 mmol/mol vs. 53 mmol/mol), p<0.0001).
15. Sherr JL, et al. Diabetes Care. 2022;45(8):1907-1910. Single-arm multicentre clinical trial in 80 pre-school children (aged 2-5.9 yrs) with T1D. Study included a 14-day standard therapy (ST) phase followed by a 3-month AID phase with Omnipod 5 system. Mean HbA1c as measured in very young children, ST vs. Omnipod 5 use: 7.4% vs. 6.9% or 57 mmol/mol vs. 53 mmol/mol, (p<0.0001). Mean time in range (3.9-10.0 mmol/L or 70-180 mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 57.2% vs. 68.1%, p<0.0001.

