How the Omnipod DASH® System may help improve glycaemic control1,2
Meeting recommended targets and day-to-day insulin management is a burden that many people living with T1D know all too well. Consisting of a lightweight*, smartphone-like Personal Diabetes Manager (PDM) and wearable Pod3, the Omnipod DASH® System has been associated with a reduction in acute complications4, and may also lead to a perceived increase in Quality of Life5.1,2
The majority of people with T1D do not meet their HbA1c targets6
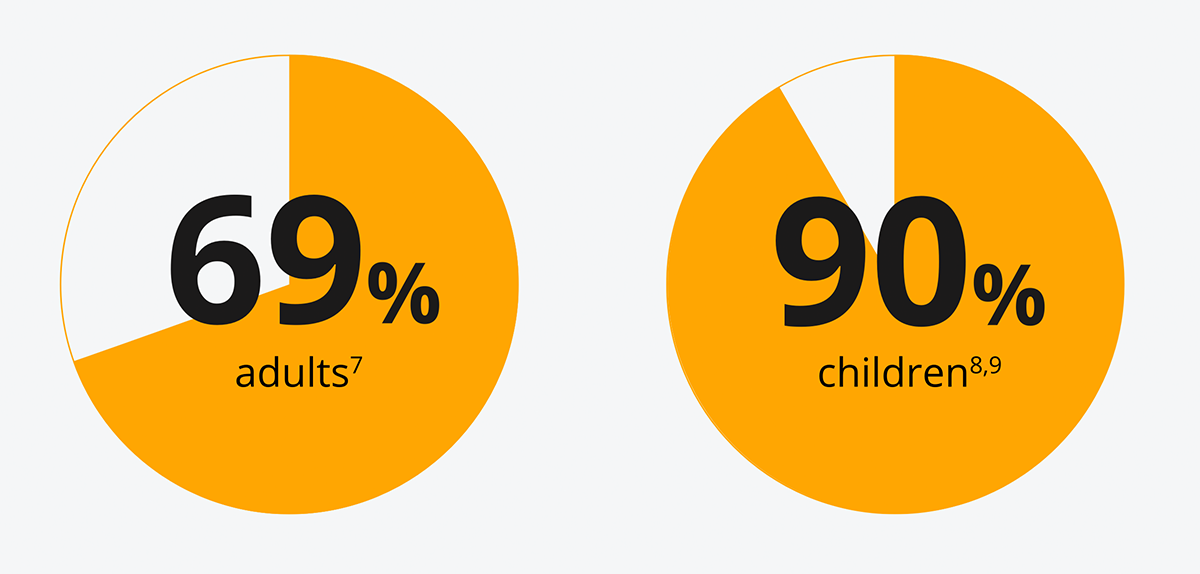

[69% of adults7 and 90% of children8,9 are not meeting recommended targets [NICE](48 mmol/mol(6.5%)].
Insufficient glycaemic control is associated with an increased risk of future complications.10,11,12
Can simplifying your patients' insulin management help them with their diabetes?
The Omnipod® System was associated with improved glycaemic control in adults with T1D1,2,12
Mean HbA1c level was significantly lower after 3, 6 and 12 months post-Omnipod® therapy, following transition from previous treatment.1
Significant improvement in HbA1c in adults with T1D, most notably in those with HbA1c ≥9.0% and those previously treated with MDI or tubed pump.1
Study description
HbA1c alone does not provide a measure of glycaemic variability or hypoglycaemia13
Even for those people living with T1D who do meet their target HbA1c, their Time in Range (TIR)* may fluctuate below and above glucose range, increasing their risk of complications and reducing their Quality of Life.14,15 Could prescribing CSII help with improvements with TIR*?
The Omnipod® System combined with a glucose sensor* improved TIR† versus multiple daily injections (MDI)+CGM results in adults with T1D15
In a randomised controlled trial, the Omnipod® System improved TIR† by an average daily proportion of 6% more than MDI without any occurrences of severe hypoglycaemia (adjusted mean difference favours CSII over MDI (95%) CI, 17-149 [p=0.01]).
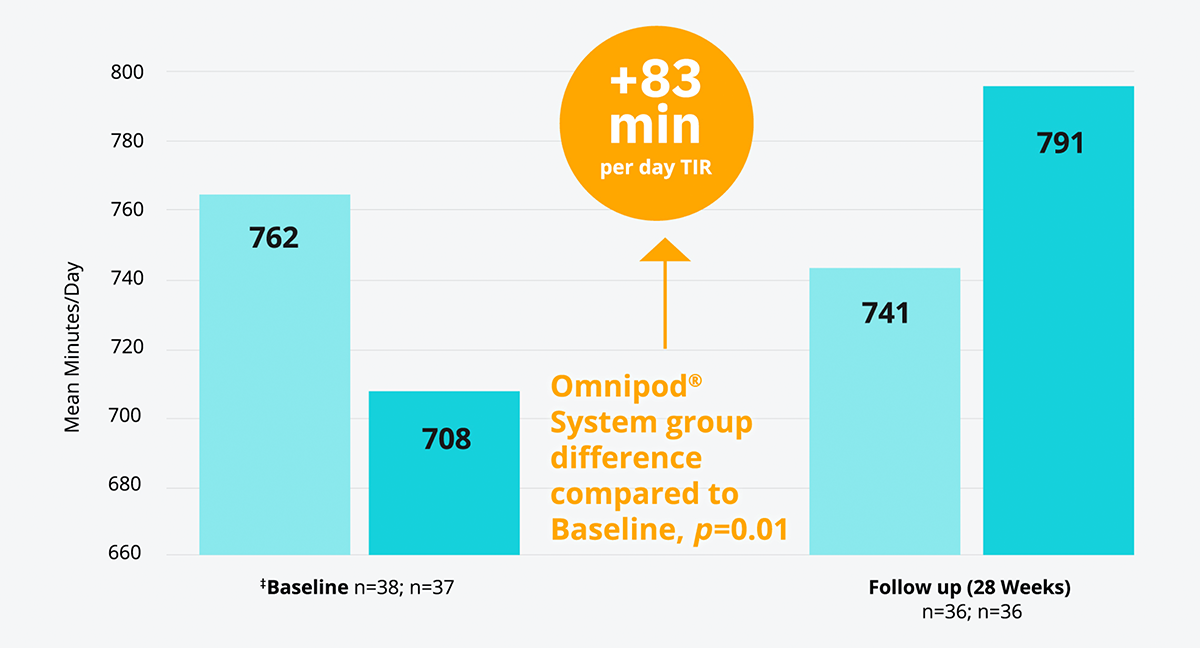

† TIR 70-180 mg/dL
‡ Baseline refers to time of randomisation for this trial; pooled follow-up combines all data collected in follow-up excluding the first 4 weeks for all participant randomly assigned, and excludes one participant in the CGM plus CSII group and two in the CGM plus MDI group with less than 72 h of follow-up data (none of whom completed the study).
The reduction in hyperglycaemia with the Omnipod® System was associated with a significant increase in CGM-measured time in hypoglycaemia compared with patients on MDI.15
- Median change in TDD = -0.18 u/kg in Omnipod® System+CGM group vs. +0.01 u/kg in MDI +CGM group; p-value <0.0001
- Severe hypoglycaemia was reported in one MDI+CGM participant; diabetic ketoacidosis and severe hyperglycaemia occurred in one participant each in the Omnipod® System+CGM group
- Increase in CGM-measured hypoglycaemia (p=0.0001 for <70 mg/dL [<3.9 mmol/L], p=0.0002 for <60 mg dL [<3.3 mmol/L], p=0·0009 for <50 mg/dL [<2.8 mmol/L], p=0.0002 for the area over the curve for 70 mg/dL [3.9 mmol/L])
Omnipod® improved TIR for patients previously on MDI15 and supported increased TIR without risk of severe hypoglycaemia.15
Study description
See the Omnipod® System data
Apart from being associated with an improvement in glycaemic control, the Omnipod® System has also been associated with a reduction in acute complications4 for patients, and a perceived increase in Quality of Life.5
Contact our Medical Affairs team
At Insulet, our aim is to keep on improving the lives of those living with type 1 diabetes. With this in mind we aim to generate real-world data to provide further evidence on the efficacy of Pod Therapy. If you want to know more about our current trials and development pipeline do not hesitate to contact our Medical Affairs team at [email protected]
Arrange a meeting with an Omnipod® representative
Do you have questions about the Omnipod DASH® System? Our team is on call to provide you with the information you need and help you to determine which of your patients could best benefit from the Omnipod DASH® System.
Be the first to know
Stay up to date and be in the know when it comes to all things Insulet. By signing up for our mailing list, you will be in the know when it comes to training and events, Omnipod® published data and first-hand experiences from Podders®.
References: 1. Mehta AN, et al. Clinical Diabetes. 2021 2. Brown RE et al.Diabet Med.2021 Jun; 38(6):e14420. doi: 10.1111/dme.14420. 3. Omnipod DASH® Insulin Management System [User Guide]. 2019. 4. Biester T, et al. Diabetes Technol Ther. 2021 5. Polonsky WH, et al. Diabetes Technol Ther. 2016;18(10):664-670. 6. Renard E, et al. Diabetes Metab Res Rev. 2021. 7. National Diabetes Audit, 2017-18. England and Wales. December 2019. 8. National Paediatric Diabetes Audit 2018/19. Care Processes and Outcomes. RCPCH March 2020. 9. NICE (2015) Diabetes (type 1 and type 2) in children and young people: diagnosis and management (NG18). 2020. NICE, London. 10. Skyler JS. Endocrinol Metab Clin North Am. 1996;25(2):243-25. 11. Renard E, et al. Diabetologia. 2019;62(suppl 1):s8-s9. 12. Renard E, et al. The SAGE study: Global observational analysis of glycaemic control, hypoglycaemia and diabetes management in T1DM. Diabetes Metab Res Rev. 2021;37(7):e3430. doi:10.1002/dmrr.3430 13. ADA. Diabetes Care, January 2021. Ch.6. 14. Battelino T, et al. Diabetes Care. 2019;42(8):1593-1603 15. Beck RW, et al. Lancet Diabetes Endocrinol. 2017;5(9):700-708.