The Omnipod 5 Automated Insulin Delivery System is a single hormone insulin delivery system intended to deliver U-100 insulin subcutaneously for the management of type 1 diabetes in persons aged 2 and older requiring insulin. The Omnipod 5 System is intended for single patient use. The Omnipod 5 System is indicated for use with NovoLog®/NovoRapid®, Humalog®, Trurapi®/Truvelog®/Insulin aspart Sanofi®, Kirsty®, and Admelog/Insulin lispro Sanofi U-100 insulin. Refer to the Omnipod® 5 Automated Insulin Delivery System User Guide and www.omnipod.com/safety for complete safety information including indications, contraindications, warnings, cautions, and instructions.
1. Insulet’s RADIANT Trial Demonstrates Meaningful Glycemic Improvements with the Omnipod® 5 Automated Insulin Delivery System Following Direct Transition from Multiple Daily Injections. March 19, 2025. Accessed June 4, 2025. https://investors.insulet.com/news/news-details/2025/Insulets-RADIANT-Trial-Demonstrates-Meaningful-Glycemic-Improvements-with-the-Omnipod-5-Automated-Insulin-Delivery-System-Following-Direct-Transition-from-Multiple-Daily-Injections/default.aspx
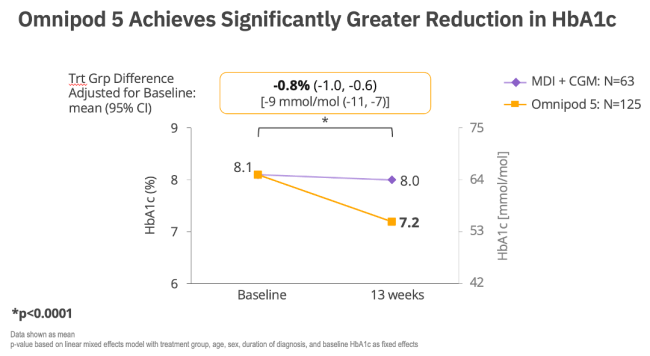
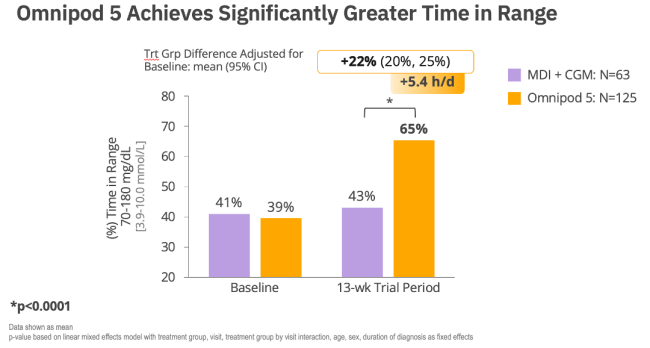
2. RADIANT Trial. Data on File. 2025. RF-042025-00015.
3. Holt R, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2021;64(12):2609-2652
4. Moshe Philip, et al. Consensus recommendations for the use of Automated Insulin Delivery (AID) technologies in clinical practice, Endocr Rev. 2023;4;44(2):254-280.