Real-World Data: Omnipod® 5 Improved Glycaemic Control

Real-World Data Supports Omnipod 5 Automated Insulin Delivery System for Improved Glycaemic Outcomes1
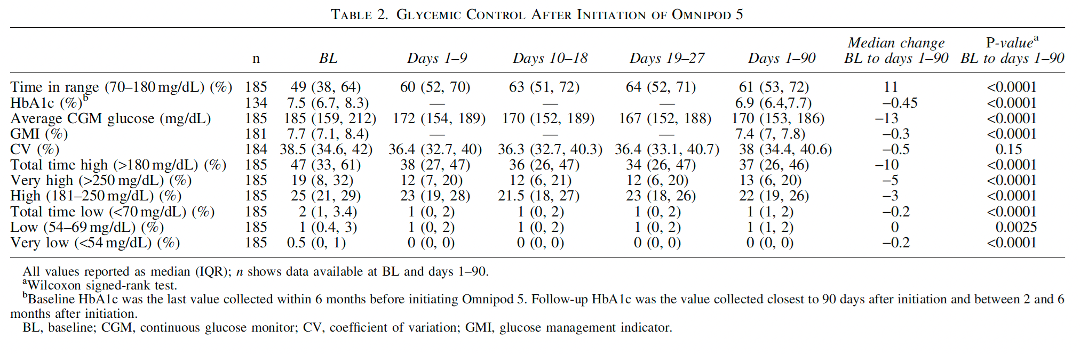
Evidence from the 2023 real-world study by Marks BE, et al. highlights the significant impact the Omnipod 5 Automated Insulin Delivery (AID) System can have for diabetes care among children, adolescents, and young adults. The study, conducted across two leading U.S. paediatric diabetes centres, followed 195 young people aged 2–21 with type 1 diabetes over the first 90 days of Omnipod 5 use.
Unlike previous pivotal trials, this research focused on a racially, ethnically, and socioeconomically diverse cohort. The results offer valuable insight into the potential of this tubeless insulin pump to transform daily diabetes care for youth.