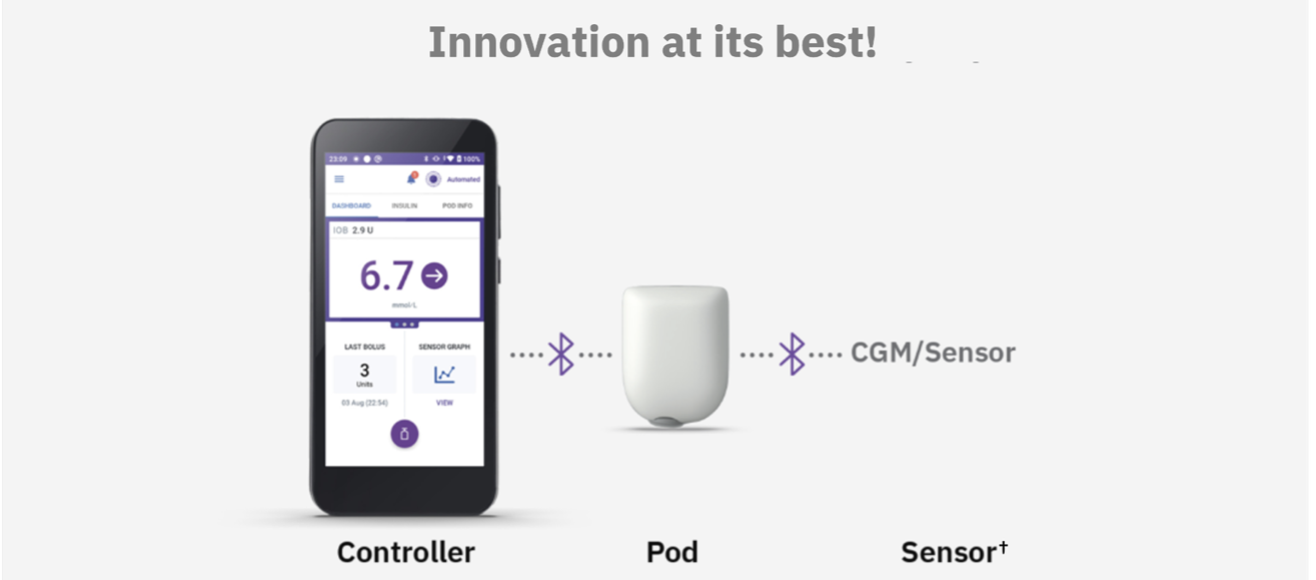
2. Understanding the Efficacy and Safety Data From the Omnipod 5 Pivotal Trials
The pivotal trials for Omnipod 5 evaluated the efficacy and safety of the system in a cohort of 320 participants aged 2 to 70 years. Across all age groups, the Omnipod 5 system demonstrated significant improvements in HbA1c and Time in Range (TIR) with minimal hypoglycaemia.1,2
Notably, Omnipod 5 patient group aged 2-5.9 years experienced a mean HbA1c reduction of 0.55% compared to standard therapy, while those aged 6-13 and 14-70 years saw reductions of 0.71% and 0.38%, respectively.1,2
In a 3-month clinical study, 3 cases of severe hypoglycaemia and 1 case of diabetic ketoacidosis (DKA) were reported in participants aged 6-70 years during Omnipod 5 System use. These cases were not related to automated insulin delivery malfunction.
Access a detailed overview of the trial results.
3. Understanding the Real-World Evidence of Performance in Daily Practice
While clinical trials provide critical insights into the efficacy of new therapies, real-world evidence offers a more comprehensive picture of how these systems function in everyday use.
In a retrospective analysis of real-world data, 6,901 Omnipod 5 users who utilised the 110 mg/dL or 6.1 mmol/L glucose target had 80% TIR or greater.*
*Forlenza G, et al. Diabetes Technol Ther (2024). Retrospective analysis of real-world data of the 37,640 Omnipod 5 users with type 1 diabetes who utilised the 110 mg/dl or 6.1 mmol/L glucose target 6,901 (18.3%) had 80% Time in Range or greater. Omnipod 5 results based on users with ≥90 days CGM data, ≥75% of days with ≥220 readings available.
Learn more about the real-world evidence for Omnipod 5.
4. Customising Glucose Targets for Your Patients
One of the key features of Omnipod 5 is the ability to set customisable glucose targets ranging from 110 to 150 mg/dL (6.1 to 8.3 mmol/L), which can be adjusted based on the patient’s daily routine.
5. Leveraging the Activity feature during Physical Activities
The Activity feature is another valuable tool for your patients who experience fluctuations in insulin needs during exercise or other physical activities. When enabled, the Activity feature raises the glucose target to 150 mg/dL (8.3 mmol/L) and reduces automated insulin delivery. You should educate patients on when and how to use this feature, particularly in preparation for exercise, as starting the Activity feature 60 to 120 minutes prior to activity to reduce automated insulin delivery during exercise.
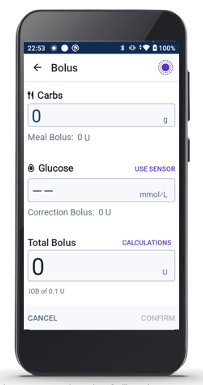
6. Ensuring Proper Bolusing at Mealtimes
While Omnipod 5 automates much of the insulin delivery process, patient engagement remains crucial, especially for bolusing at mealtimes. The SmartBolus Calculator, which considers sensor trends, carbohydrate intake, and insulin on board, helps patients determine the appropriate bolus dose. This is an important feature in improving your patient’s glycaemic control.