Omnipod® 5 Automated Insulin Delivery System

Omnipod 5 is the first wearable, tubeless, hybrid closed loop system that integrates with the leading sensor brands, for people with type 1 diabetes aged 2 years and older.
The Omnipod 5 System works with the Dexcom G6 and FreeStyle Libre 2 Plus sensors to continuously adjust, correct, and automatically deliver basal insulin according to your patients’ needs.
The System - 3 Simple Parts
Controller + Pod + Sensor
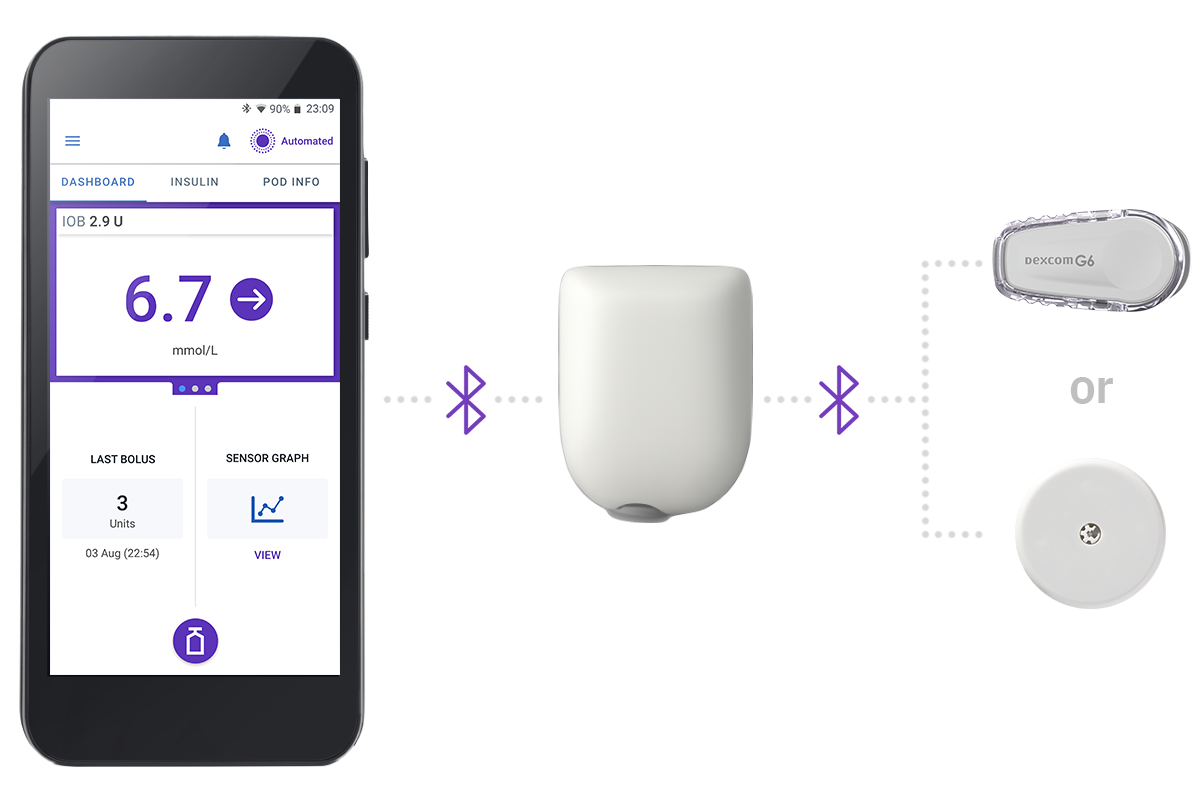

Omnipod 5 Controller
Take control of the system with the Omnipod 5 Controller. Monitor and control the Pod using Bluetooth® wireless technology.

Pod
Tubeless, wearable, and waterproof,** the Pod, with built-in SmartAdjust™ technology, sits right on your patient’s body and automatically adjusts insulin delivery for up to 3 days or 72 hours.


Sensor
Continuously sends glucose values to the Pod, so your patients can get real-time data† without the finger pricks†.


SmartAdjust™ Technology
Predicts
Glucose 60 minutes into the future
Adjusts
Insulin delivery using the selected glucose target
Delivers
Insulin doses every 5 minutes (as needed)
SmartAdjust™ technology is at the heart of Omnipod 5, proactively managing insulin delivery every 5 minutes. It automatically increases, decreases or pauses insulin delivery to your patient’s personal needs which may help to prevent against highs and lows.1,2
Watch this video to learn more.
Proven Clinical Outcomes
Omnipod 5 improved glycaemic control in adults, adolescents and children with T1D in clinical studies.1-3
Time in range
76% of time in range for adults and adolescents (target 110mg/dL; 6.1 mmol/L)
68% of total time in range for children (2-13.9 years)1-3
HbA1c
significantly reduced by 0.5% in very young children (2.0-5.9 years), 0.7% in children (6-13.9 years) and 0.4% in adults and adolescents (14-70 years)2,3
Hyperglycemia
33% reduction in time spent in hyperglycemia in children and 24% in adults and adolescents2,3
Hypoglycemia
60% reduction in time spent in hypoglycemia during the night in adults and adolescents and 46% reduction in total time2
Safety: There were 3 severe hypoglycaemia and 1 DKA events during the pivotal study. These events were not related to automated insulin delivery malfunction.1,2
Omnipod® 5 improved users’ quality of life in clinical studies4
- Improved diabetes distress
- Quality of life
- Less stress when eating
- Improved confidence in managing diabetes
1. Brown et al. Study in 128 people with T1D aged 14 - 70 years average time in Target Glucose range (from CGM) during 110mg/dL Target BG in adults/adolescents (n = 121) = 75.6%. Diabetes Care (2021).
2. Brown et al. Study in 240 people with T1D aged 6 - 70 years involving 2 weeks standard diabetes therapy followed by 3 months Omnipod 5 use in Automated Mode. Average A1c in adults/adolescents and children, standard therapy vs. Omnipod 5 = 7.16% vs 6.78%; 7.67% vs 6.99%. Average time with high blood glucose (from CGM) in adults/adolescents and children, standard therapy vs. omnipod 5, 32.4% vs. 24.7%; 45.3% vs. 30.2% respectively. Average time with low blood glucose (12AM - <6AM) from CGM in adults/adolescents and children, standard therapy vs 3-month Omniopod = 2.07% vs. 0.82%; 0.78% vs. 0.78%. Overall median time with low blood glucose (from CGM) in adults/adolescents and children for standard therapy vs Omnipod 5 = 2.00% vs 1.09%; 0.78% vs. 0.78%; 1.38% vs. 1.48%. Diabetes Care (2021).
3. Sherr JL, et al. Study in 80 people with T1D aged 2 - 5.9 yrs involving 2 weeks standard diabetes therapy followed by 3 months Omnipod 5 use in Automated Mode. Average time in Target Glucose range (from CGM) for standard therapy vs Omnipod 5 = 57.2% vs. 68.1%. Average A1c in standard therapy vs. Omnipod 5 = 7.4% vs. 6.9%. Diabetes Care (2022).
4. Polonsky et al. Study in 115 people with T1D aged 18–70 years involving 2 weeks standard diabetes therapy followed by 3 months Omnipod 5 use in Automated Mode. Diabetes Distress measured by Type 1 Diabetes Distress Scale (T1-DDS) 1.64 vs. 1.48 (P=0.0001). Eating Distress measured by Type 1 Diabetes Distress Scale (T1-DDS) subset score 1.97 vs. 1.73 (P=0.0003) respectively. Hypoglycemia confidence measured by (HCS) 3.52 vs. 3.65 (P=0.0002). Diabetes Res Clin Pract (2022).
Initiating a Patient on Omnipod 5
Help your patients get a successful start on Omnipod® 5.
Omnipod 5 Simplifies Data Management
With Glooko®, your patient can access all of their diabetes information in one easy-to-use platform and easily share it with you.
Omnipod 5 Resources for your Patients
All of the resources, all in one place. Visit our resources page for User Guides, How-to-Videos, FAQs and more.
**The Pod has an IP28 rating for up to 7.6 metres (25 feet) for up to 60 minutes. The Omnipod 5 Controller is not waterproof. Please consult sensor manufacturer user guide for sensor waterproof rating.
†Fingerpricks required for diabetes treatment decisions if symptoms or expectations do not match readings.
Important Safety Information: The Omnipod® 5 Automated Insulin Delivery System is indicated for use by individuals with Type 1 diabetes mellitus in persons 2 years of age and older. The Omnipod 5 System is intended for single patient, home use and requires a prescription and/or ongoing supervision of a qualified healthcare provider. The Omnipod 5 System is compatible with the following U-100 insulins: NovoLog®/ Novorapid®, Humalog®, Trurapi®/Insulin aspart Sanofi®, Kirsty®, and Admelog®/Insulin lispro Sanofi®.
Refer to the Omnipod 5 Automated Insulin Delivery System User Guide and www.omnipod.com/safety for complete safety information including indications, contraindications, warnings, cautions, and instructions.
Warning: DO NOT start to use the Omnipod 5 System or change settings without adequate training and guidance from a healthcare provider. Initiating and adjusting settings incorrectly can result in over-delivery or under-delivery of insulin, which could lead to hypoglycaemia or hyperglycaemia.