Article Review: Commissariat et al, 2017
Article Review
Written by: Lucy Casson RN, CDE, MA(Nursing)
Paediatric Diabetes Nurse Practitioner
Lucy Casson received financial support for the writing of this material. Her opinion is solely her own.
The Article “Insulin Pump Use in Young Children with Type 1 Diabetes: Sociodemographic Factors and Parent-Reported Barriers” (Commissariat et al, 2017) ¹ addresses the many reasons against the choice of insulin pump therapy for young children. Whilst published in 2017, sadly many of these issues are still relevant today. Despite the wealth of research supporting the use of technology, particularly in this age group² the uptake is not what it should or could be. Many of these are also experienced in my own clinical practice.
Parent perceptions of physical burden (citing those associated with tubing as one reason) are outweighing the benefits of therapy effectiveness [table 3, below], even when encouraged by their HCP to use it.
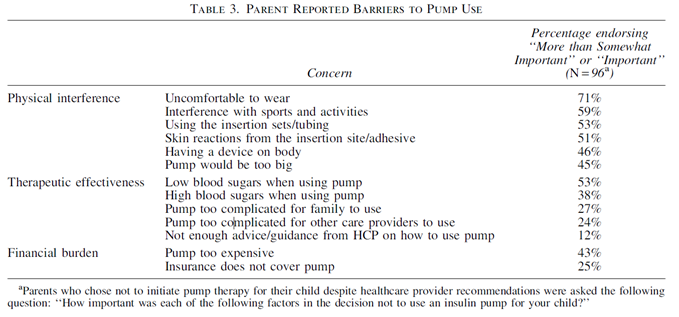

Despite this encouragement, parents felt the physical burden outweighed the clinical benefit, thus highlighting the importance for the HCPs to discuss all therapy options.
Discussion:
This article potentially raises the question of HCP tech bias and a lack of choice of technology to suit the family's need? These factors are highlighted below.
- Reducing the barrier of unconscious HCP bias
Despite evidence to support the use of diabetes technology, some health care professionals still demonstrate a degree of unconscious bias about who should be offered or recommended insulin pump therapy. Some examples of this unconscious bias are perceived intellectual ability, language barriers, financial constraints and whether they are currently engaged with technology, for example, CGM. Current research states that our assumptions are in fact incorrect and often, the patients who are having the poorest glycaemic outcomes on injection therapy, may achieve the greatest improvements when technology is introduced3,4. This should present sufficient reason to change the practice of those health care professionals still only offering or recommending insulin pump therapy to those families that they feel ‘meet their criteria’. We should be offering a variety of diabetes technology options to all, as barriers such as cost are reduced. CGM subsidy for all ages has now been introduced and new payment models for Insulin Pumps have been developed.
- Standard of care or ‘luxury extra’?
International research and diabetes governing bodies all agree that technology delivers the best clinical outcomes⁵, therefore we need to be using this evidence to lead our decision making. As clinicians, I believe we need to shift our view away from thinking of diabetes technology as an ‘advanced’ way of managing diabetes and start thinking of it as our industry standard.
- Myths and fears.
Our job as HCP’s is to dispel fears, myths and perceived issues around living with diabetes technology. I often see in my practice that expectations and perceptions of diabetes technology may be worse than the lived experience. As a Nurse Practitioner, providing counselling support for parents, helps them to make the best choices for their child.
- Reducing the barriers of cost and financial bias
Financial constraints are a recognised barrier to pump uptake, as per Table 3. Providing choice in access, alongside product choice is important to families who often have the dilemma of having PHI, versus not committing to a product for 4 years. Subsidy exists for both PHI and personal pay pathways with Omnipod DASH® Insulin Management System. I have a number of families who have taken advantage of these options by using their health insurance, once confident in their decision. This is a unique option with Omnipod DASH®, which also justifies it as an option for lower income families, or those without health insurance.
- Does choice matter?
Standards of care recommend the following for patients living with type 1 diabetes: “the type(s) and selection of devices should be individualised based on a person’s specific needs, preferences, and skill level”6. Wearability of a device as a preference is an example of choices that patients can make and should be supported. A structured decision-making process helps to navigate needs, preferences, and skill level, in the discussion between HCP and patient.
In my practice there are patients for whom a tubed pump was simply not an option for various reasons (lifestyle, practicality, and wearability). By providing a choice of Omnipod DASH® System, these patients preferences to match their lifestyle were achieved, in addition to meeting their clinical goals. In a continuously evolving technology landscape, it can be difficult to be unbiased in the decision-making process. Ultimately, patients will seek out what they want anyway, and I believe that as HCPs, we need to keep up to date in this fast-evolving space to support choice.
1) P. V. Commissariat et al
Insulin Pump Use in Young Children with Type 1 Diabetes: Sociodemographic Factors and Parent-Reported Barriers. DIABETES TECHNOLOGY & THERAPEUTICS
Volume 19, Number 6, 2017
2) J. L. Sherr, M. Schoelwer, T. Jeronimo Dos Santos, L. Reddy, T. Biester, A.Galderisi, J. Cornelius van Dyk, M. E. Hilliard, C. Berget, L. A. DiMeglio
ISPAD Clinical Practice Consensus Guidelines 2022: Diabetes technologies: Insulin Delivery.
Pages: 1406-1431 First Published: 05 December 2022
3) L.L Snyder, J.M Stafford, D. Dabelea, J. Divers, G. Imperatore, J. Law, J.M. Lawrence, C. Pihoker, E.J. Mayer-Davis
Socio-economic, demographic, and clinical correlates of poor glycaemic control within insulin regimens among children with Type 1 diabetes: the SEARCH for Diabetes in Youth Study. Diabetic Medicine: Pages: 1028-1036 First published: 02 May 2019
4) P. Di Bartolo, A. Nicolucci , V. Cherubini , et al.
Young patients with type 1 diabetes poorly controlled and poorly compliant with self-monitoring of blood glucose: can technology help? Results of the i-NewTrend randomized clinical trial. Acta Diabetol: 54: 393- 402 Published 2017
5) R. Cardona-Hernandez, A. Schwandt , H. Alkandari , et al.
Glycemic outcome associated with insulin pump and glucose sensor use in children and adolescents with type 1 diabetes. Data from the international pediatric registry SWEET. Diabetes Care: 44: Pages: 1176- 1184 Published 2021
6) 2023: ADA Standards of Care , Diabetes Care 2023;46(Suppl. 1):S111–S127 https://doi.org/10.2337/dc23-S007
© 2023 Insulet Corporation. Omnipod, the Omnipod logo, DASH, the DASH logo, Podder and Simplify Life are trademarks or registered trademarks of Insulet Corporation. All rights reserved. The Bluetooth® word mark and logos are registered trademarks owned by the Bluetooth SIG, Inc. and any use of such marks by Insulet Corporation is under license. Glooko is a trademark of Glooko, Inc. and used with permission. All other trademarks are the property of their respective owners. The use of third-party trademarks does not constitute an endorsement or imply a relationship or other affiliation. Insulet Australia PTY LTD Level 16, Tower 2 Darling Park,201 Sussex Street, Sydney, NSW 2000 INS-ODS-06-2023-00030 V1.0