Experience Freedom with Pod Therapy
A simple and discreet way to deliver insulin.

What is Pod Therapy?
Pod Therapy is simple, tubeless, discreet insulin pump therapy for people living with type 1 diabetes.
Each wearable Pod continuously delivers personalised doses of insulin for up to three days (72 hours), controlled wirelessly by you, wherever you are.
With no multiple daily injections and no tubes, Pod Therapy is insulin pump therapy, simplified.
Meet Omnipod DASH®


Omnipod DASH® Insulin Management System
You’re in control with the Omnipod DASH® personal diabetes manager. Discreetly and conveniently adjust your insulin doses, anywhere you are.

THE PERSONAL DIABETES MANAGER (PDM)
Using your Omnipod DASH® Personal Diabetes Manager (PDM) you can set various presets to establish favourites and tag your activities and personalise insulin delivery based on your daily routine.
THE POD
A tubeless, wearable Pod that is waterproof‡ and discreet (can be worn under clothing), which is controlled§ by the Omnipod DASH® Personal Diabetes Manager (PDM).
§ At start-up, the Pod and Personal Diabetes Manager must be adjacent and touching. During normal operation, the Personal Diabetes Manager must be within 1.5 meters (5 feet) of the Pod.
How does the Omnipod DASH® System work?
With the Omnipod DASH® System, you have an insulin management system that can provide up to 72 hours§ of continuous insulin delivery. It all starts with a tubeless, wearable Pod that is waterproof‡ and discreet (can be worn under clothing), which is controlled◊ by the Omnipod DASH® Personal Diabetes Manager (PDM). Using your Omnipod DASH® PDM you can set various presets to establish favourites and tag your activities and personalise insulin delivery based on your daily routine.
†The Pod has a waterproof IP28 rating for up to 7.6 metres for 60 minutes. The PDM is not waterproof.
Learn about the Omnipod DASH® System
How does the Omnipod DASH® System work?
You can wear the Pod almost anywhere you would administer an insulin injection. Placement is easy and the cannula inserts automatically and insulin delivery begins with a few taps of a touchscreen.
You use the Omnipod DASH® PDM to conveniently and discreetly communicate§ with your Pod, adjusting insulin doses anywhere you are.
Please consult with your healthcare provider to understand if the Omnipod DASH® System would be a suitable treatment option.
The PDM
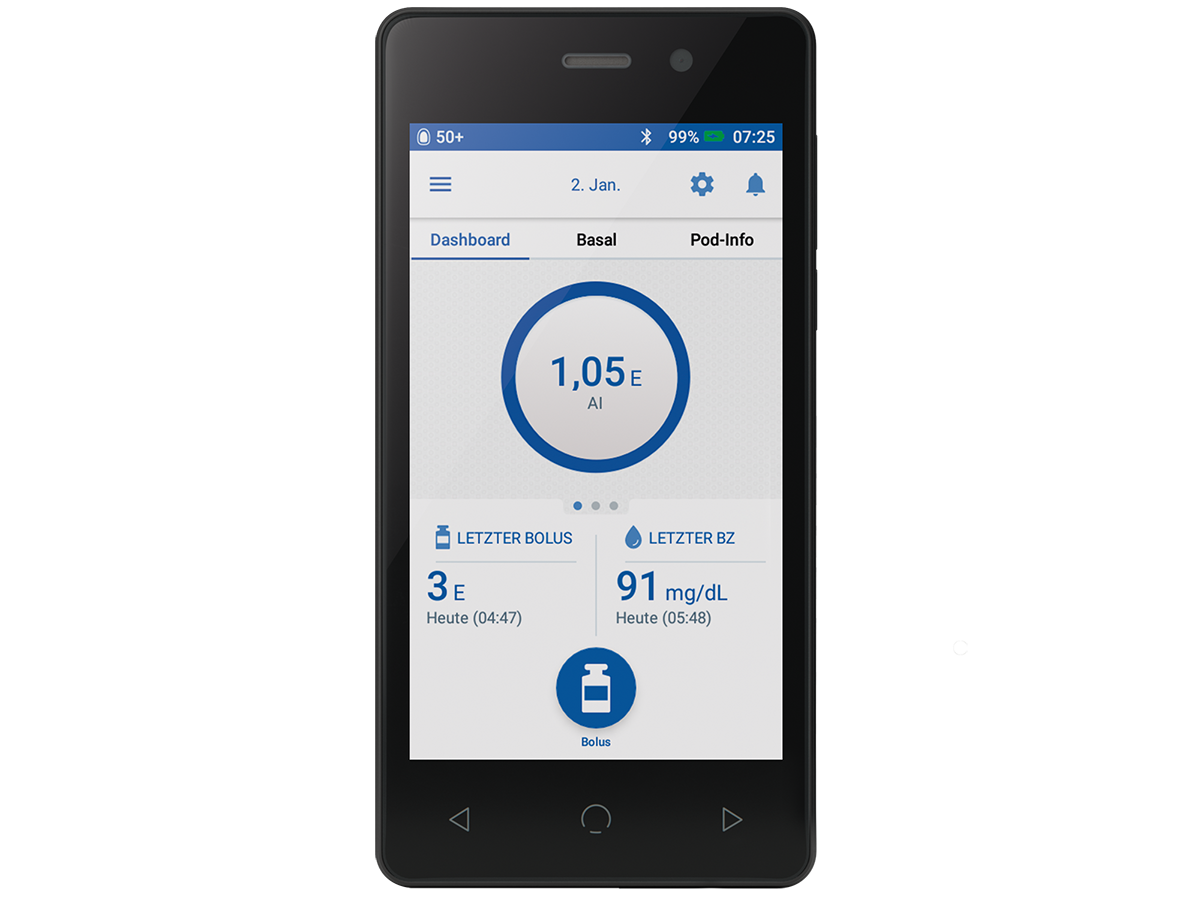

Multiple Omnipod DASH® PDM features support how you control your insulin
- You can set a Zero Basal Rate if you require low insulin delivery
- You can set Fractional Insulin to Carb Ratios (0.1g/U) if you are insulin sensitive
- The Pod Site Tracker helps you to track your Pod site locations.
- You can set various presets on the Omnipod DASH® PDM to establish favourites, tag your activities and personalise insulin delivery based on your daily routine
Compatibility with other systems simplifies the tracking and recording of data
Insulet Provided diasend® allows you to review your diabetes patterns on your smartphone or personal computer, understand the cause-and-effect relationships of your activities on your blood glucose, and easily share diabetes data with your diabetes healthcare provider.
The Pod


The Pod is a small, tubeless, wearable and waterproof† device that you fill with insulin and wear directly on your body.
Its durable, waterproof† exterior shell houses a built-in 200-unit insulin reservoir, angled infusion set, automatic inserter, delivery mechanism and power supply. You can place the Pod almost anywhere you would normally give yourself an injection, for up to three days of non-stop insulin delivery‡.
‡Up to 72 hours of insulin delivery.
Refer to the Omnipod DASH® Insulin Management System User Guide for complete safety information, including indications, contraindications, warnings, cautions and safety instructions.
The Pod includes a small, flexible cannula that inserts automatically with the push of a button.
It delivers personalised doses of insulin into your body based on the set and variable rates that you program into its handheld companion, the Omnipod DASH® PDM.
The Pod communicates wirelessly§ with the Omnipod DASH® PDM to program insulin delivery.
The Pod can be worn comfortably and discreetly under your clothes, while carrying the Omnipod DASH® PDM separately in a pocket, backpack or handbag.
Three simple steps to insulin delivery
Step 1. Fill the Pod.
The Pod is paired with the PDM and automatically primes with the touch of a button.
Step 2. Apply the Pod.
Place your Pod almost anywhere you would give yourself an insulin injection.
Step 3. Activate Insulin Delivery.
The cannula inserts automatically and insulin delivery begins with a few taps of a touchscreen.
Refer to the Omnipod DASH® Insulin Management System User Guide for complete safety information, including indications, contraindications, warnings, cautions and safety instructions.
Learn about the Omnipod DASH® System
How can I find out more about the Omnipod DASH® System?
We are delighted that the Omnipod DASH® System is available in Saudi Arabia. Please contact our distributor MediServ to learn more.
Address: Riyadh
Al Ihsa Street
Az Zahra
MediServ HQ Building
Web site: www.mediserv.com
Email: [email protected]
Phone: 0114780555 ext. 1181 or Mobile Number: 00966558491266
Omnipod 5 is a wearable, tubeless, hybrid closed loop system that integrates with the leading sensor brands, for people with type 1 diabetes aged 2 years and older requiring insulin. It will be available for your patients in 2026.
Three Simple Parts
Controller + Pod + Sensor*


Omnipod 5 Controller
The Omnipod 5 Controller allows you to monitor and control the Pod using Bluetooth® wireless technology.
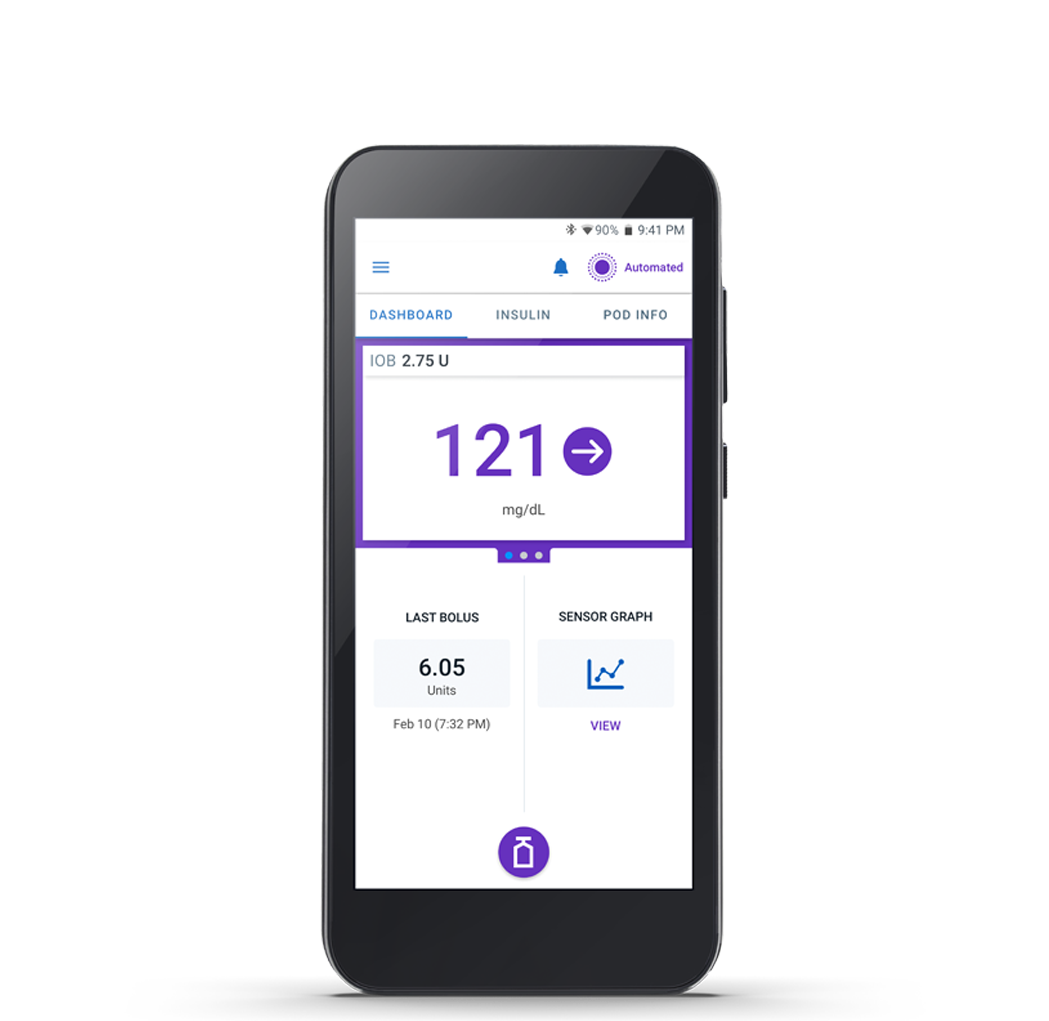

Pod
Tubeless, wearable, and waterproof,** the Pod, with built-in SmartAdjust™ technology, sits right on your patient’s body and automatically§ adjusts insulin delivery for up to 3 days (72 hours).
§Automated Mode requires compatible CGM/Sensor
**The Pod has an IP28 rating for up to 7.6 metres for up to 60 minutes. The Omnipod 5 Controller is not waterproof. Please consult sensor manufacturer user guide for sensor waterproof rating.


Sensor
Continuously sends glucose values to the Pod, so your patients can get the real-time dataƗ without the finger pricks†.
†Fingerpricks required for diabetes treatment decisions if symptoms or expectations do not match readings.


SmartAdjust™ so you don’t have to!1
Omnipod 5 with SmartAdjust™ technology automatically adjusts to your patients’ personal needs by increasing, decreasing, or pausing insulin delivery every five minutes – which may help prevent highs and lows.2,3
Diabetes Data Management, Simplified
Omnipod Discover is a simplified diabetes data management system that lets you and your Omnipod 5 patients see how their pump setting and bolus behavior is impacting their blood glucose levels—empowering you to develop diabetes management strategies together.
Find answers to the most frequently asked questions on Omnipod 5
What is Omnipod 5?
Omnipod 5 is a wearable, on-body, tubeless, hybrid closed loop system integrated with compatible sensors, cleared in Saudi Arabia for use in people with Type 1 diabetes, aged 2 and above. It works with compatible sensors to continuously adapt and automatically deliver insulin according to the user's personal needs.
How does Omnipod 5 differ from Omnipod DASH®?
Omnipod 5 works with compatible sensors to continuously adapt and automatically deliver insulin according to the user's personal needs. Omnipod DASH is not integrated with any sensor and is not capable of automated insulin delivery, but rather delivers insulin based on the user's programmed basal rates.
When will Omnipod 5 be available in Saudi Arabia?
Omnipod 5 is now available in several countries across the globe. Building on these successful recent launches, Insulet intends to launch Omnipod 5 more broadly in select countries across the Middle East starting in 2026. To be kept up to date with product information, sign up for our Interest List and we will be in touch with you to provide further details.
Who can use Omnipod 5?
In Saudi Arabia Omnipod 5 has been approved for use for ages 2 years and over, for people with Type 1 diabetes requiring insulin.
*Sensors are sold separately and each require a separate prescription.
1.In Automated Mode, SmartAdjust™ technology uses your total daily insulin (TDI) to set a new Adaptive Basal Rate for you. Requires a compatible sensor. Compatible sensor prescribed and sold separately.
2. Brown S. et al. Diabetes Care. 2021;44:1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6 - 70 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time in hyperglycaemic range (>10.0 mmol/L or >180mg/dL) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 28.9% vs. 22.8%; 44.8% vs 29.7%, P<0.0001, respectively. Mean time in hypoglycaemic range (<3.9 mmol/L or <70 mg/dL) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 2.89% vs. 1.32%, P<0.0001; 2.21% vs. 1.78, P=0.8153, respectively.
3. Sherr J. et al. Diabetes Care. 2022; 45:1907-1910. Single-arm multicenter clinical trial in 80 pre-school children (aged 2-5.9 yrs) with T1D. Study included a 14-day standard therapy (ST) phase followed by a 3-month AID phase with Omnipod 5 system. Mean time in hyperglycaemic range (>10.0 mmol/L or Stay in the know! Sign up here to get the latest news and updates. Find answers to the most frequently asked questions on Omnipod 5 >180mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 39.4% vs. 29.5%, P<0.0001, respectively. Mean time in hypoglycaemic range (<3.9 mmol/L or <70 mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 3.43% vs. 2.46%, P=0.0204.
Important Safety Information: The Omnipod 5 Automated Insulin Delivery System is a single hormone insulin delivery system intended to deliver U-100 insulin subcutaneously for the management of type 1 diabetes in persons aged 2 and older requiring insulin. The Omnipod 5 System is intended for single patient use. The Omnipod 5 System is indicated for use with NovoLog®/NovoRapid®, Humalog® /Liprolog ®, Trurapi®/Truvelog®/Insulin aspart Sanofi®, Kirsty®, and Admelog/Insulin lispro Sanofi U-100 insulin. Warning: SmartAdjust technology should not be used by anyone under the age of 2 years old or by people who require less than 5 units of insulin per day as the safety of the technology has not been evaluated in this population.
Refer to the Omnipod® 5 Automated Insulin Delivery System User Guide and www.omnipod.com/en-gb/safety for complete safety information including indications, contraindications, warnings, cautions, and instructions.