Simplify Life with Omnipod® 5


No multiple daily injections or finger prick testing‡. Omnipod 5 proactively helps to correct highs and protect from lows1,2 –simplify your diabetes management.
How it works
Every five minutes the glucose Sensor reports glucose levels to the Pod. SmartAdjust™ technology within the Pod automatically adjusts your insulin.
- When glucose levels are dropping, insulin delivery automatically decreases or pauses.
- When glucose levels are rising, insulin delivery automatically increases.
Get to know the Omnipod 5 System


The Pod
Tubeless, wearable, and waterproof†, each Pod sits comfortably on the body for up to 3 days (72 hours), automatically adjusting insulin delivery thanks to its built-in SmartAdjust™ technology.


The Controller
Take charge of your diabetes with the handheld Omnipod 5 Controller, which connects seamlessly to your Pod via Bluetooth® for discreet, wireless control.


The Sensor
Your choice of compatible Sensor continuously sends glucose values to the Pod, giving you real-time readings§ without the hassle of finger pricks‡. The Pod and Sensor need to be in ‘line of sight’ to stay in Automated Mode. Placing them on the same side of the body allows for best communication between the devices.
Connect to Omnipod Discover™
Omnipod Discover is the diabetes data management system that connects to Omnipod 5. By connecting your Omnipod 5 account with Omnipod Discover you have the ability to share your data with your healthcare professional allowing you to:
- Review your personalised diabetes insights including insulin delivery insights, trends and patterns
- Share data with your Healthcare professional and/or diabetes clinic
- Receive weekly detailed reports from Discover providing overall insulin usage and readings from your glucose monitoring sensor
3 simple parts. One continuous loop of communication.
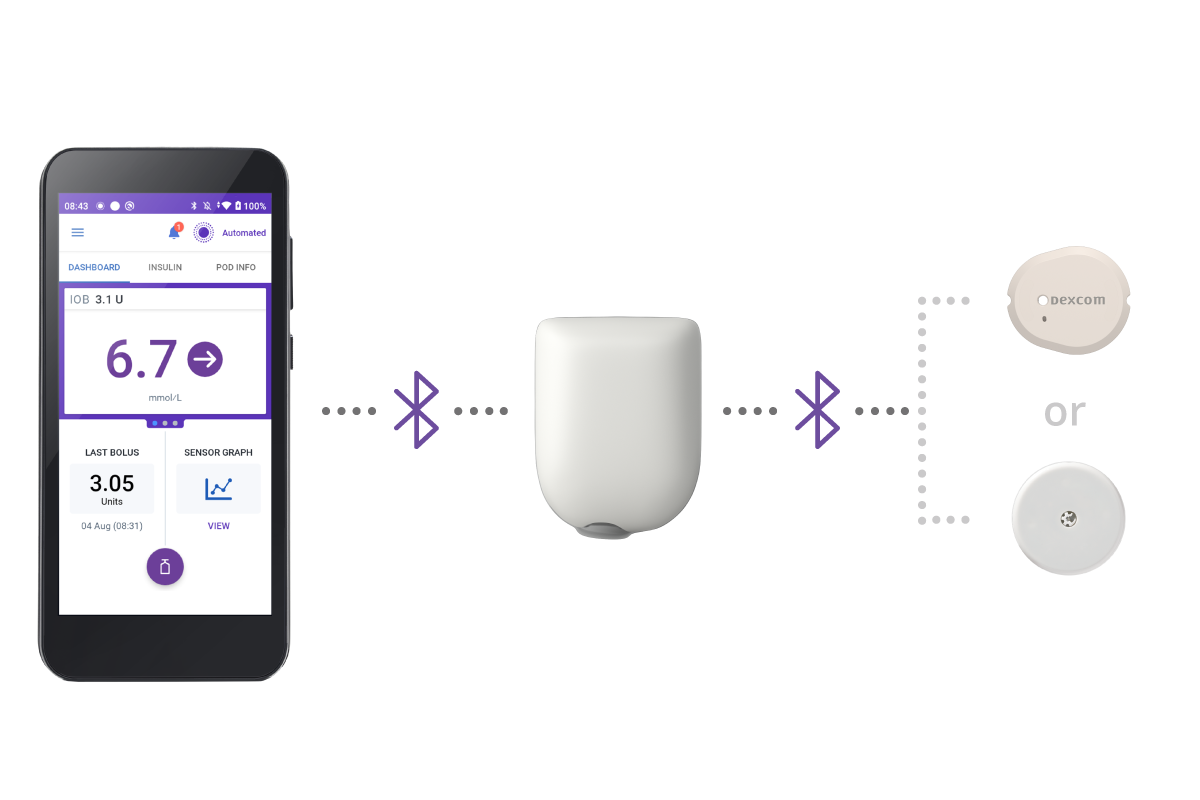

When paired with a compatible Continuous Glucose Monitor (CGM) sensor, Omnipod 5 can give constant and automatic insulin delivery to keep you in range, day and night2-3. Omnipod 5 is compatible with Dexcom G7, and FreeStyle Libre 2 Plus Sensor..
Here’s what our Podders have to say about Omnipod®:
Omnipod 5 has allowed me to get a good night sleep. That's the first time I can say that in a long time.
Alvin
Podder since 2017
It helps me feel like a normal kid, just with a little bit of help.
Romey T.
Sponsored Omnipod® User
& Podder since 2019
I don’t have to spend as much time thinking about diabetes.
Clare F.
Podder® since 2013
Speak with your healthcare provider to find out more!
Looking for Omnipod resources?
To learn more about Omnipod 5, check out our library of resources.
‡Fingersticks required for diabetes treatment decisions if symptoms or expectations do not match readings.
†The Pod has an IP28 rating for up to 25 feet for 60 minutes. The Controller is not waterproof.
1. The Omnipod 5 System was named 2023 Patient Choice Award Winner for preferred pump in the United States; Source: Seagrove Patient Perspectives Survey Report (413 Total Responses, 260 T1D, 153 T2D 245 pumpers, 98 MDI, 70 non-MDI). Seagrove Partners, LLC Patient Perspectives Report, December 2023. Insulet data on file.
2. Brown S. et al. Diabetes Care (2021). Study in 240 people with T1D aged 6 70 years involving 2 weeks standard diabetes therapy followed by 3 months Omnipod 5 use in Automated Mode. Average time with high blood glucose in adults/adolescents and children, standard therapy vs 3-month Omnipod 5: 32.4% vs. 24.7%; 45.3% vs. 30.2%. Average time with low blood glucose in adults/adolescents and children, standard therapy vs 3-month Omnipod 5: 2.9% vs. 1.3%; 2.2% vs. 1.8%. Study funded by Insulet.
3. Sherr JL, et al. Diabetes Care (2022). Study in 80 people with T1D aged 2 5.9 yrs involving 2 weeks standard diabetes therapy followed by 3 months Omnipod 5 use in Automated Mode. Average time with high blood glucose in very young children, standard therapy vs 3-month Omnipod 5: 39.4% vs. 29.5%. Average time with low blood glucose in very young children, standard therapy vs 3-month Omnipod 5: 3.41% vs. 2.13%. Study funded by Insulet.