About Omnipod® Products
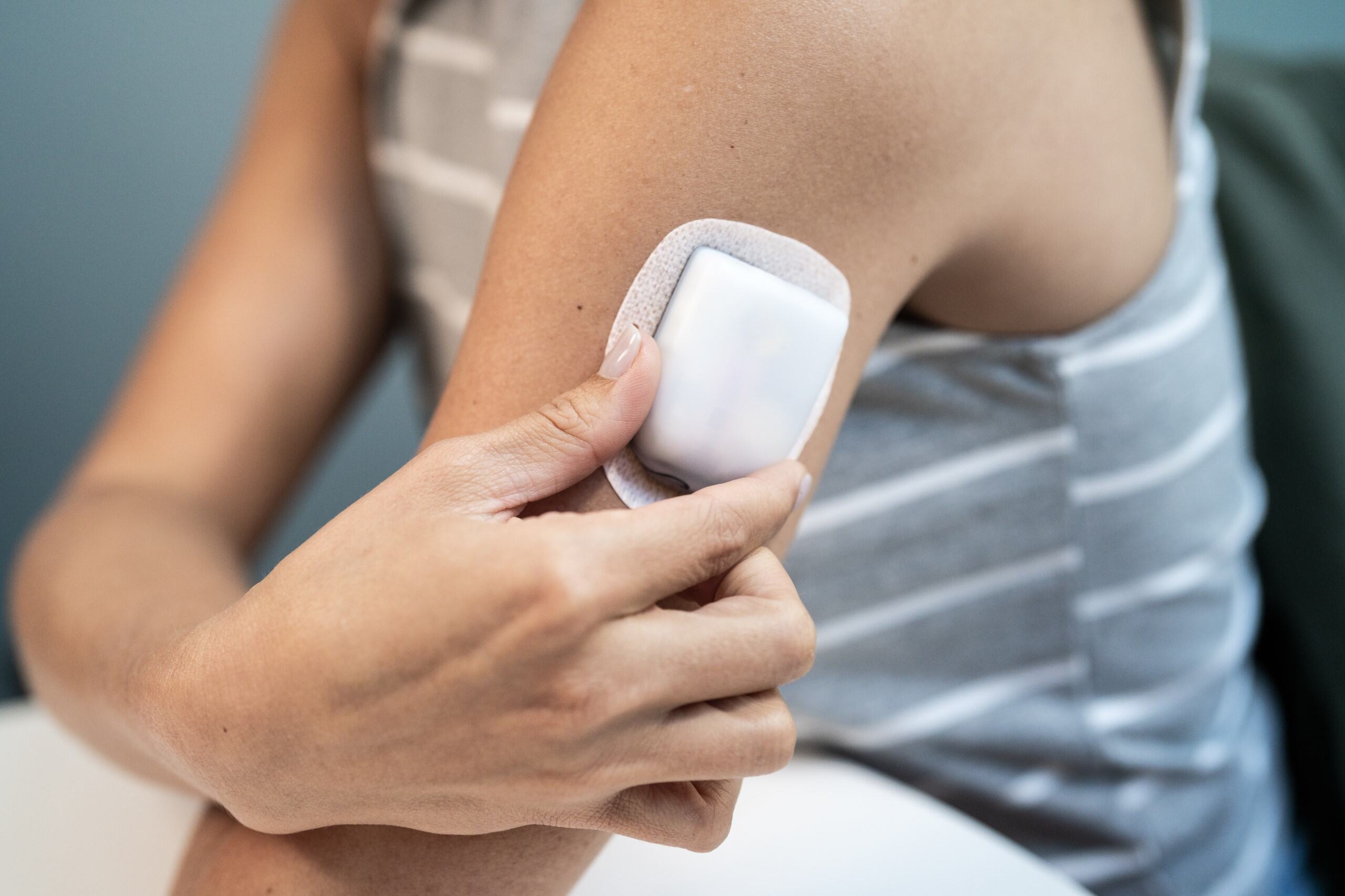
The Omnipod® 5 Automated Insulin Delivery System and the Omnipod DASH® Insulin Management System
Omnipod® combines a small, tubeless, waterproof*, wearable device (the Pod) with a handheld device, the Personal Diabetes Manager (PDM), also called a Controller. Fill the Pod with insulin and it can be worn directly on the body almost anywhere you would administer an insulin injection. The Pod communicates wirelessly† with the PDM/Controller to program insulin delivery.
Whether your patient is newly diagnosed with diabetes, currently receives daily insulin injections, or is considering a switch from another insulin pump, one of our Omnipod products may be the right insulin management option.
*The Pod has a waterproof IP28 rating for up to 7.6 metres (25 feet) for 60 minutes. The PDM/Controller is not waterproof.
Introducing Omnipod® 5 Automated Insulin Delivery System


- A wearable, tubeless automated insulin delivery (AID) system compatible with Dexcom G7 sensor
- Built-in SmartAdjust™ technology adjusts basal insulin so you don’t have to and helps to correct highs and protect from lows1-3
- Customisable glucose targets can accommodate your patients’ routines
- SmartBolus calculator is informed with sensor value and trend for bolus recommendations
- Now featuring Omnipod Discover™ management system
Diabetes data management, simplified
Experience a smarter way to support your Omnipod® 5 patients. With sensor data, insulin, carbs, usage metrics, and Pod settings all in one place—plus a seamless cloud connection, Omnipod Discover™ delivers simple diabetes data all in one report. It continually and automatically collects Omnipod 5 and sensor data wirelessly to help track glucose trends, insulin delivery data, and pump settings all in one report.
So you can optimise your time with your Omnipod 5 patients.
Introducing Omnipod DASH® Insulin Management System

Screen image is an example, for illustrative purposes only.
- Pod therapy may help simplify life for your patients with type 1 diabetes, by providing an alternative to Multiple Daily Injections (MDI)
- It’s tubeless, waterproofΩ and wearable
- Set a Zero Basal Rate for time when low insulin delivery is needed
- Set Fractional Insulin to Carb Ratios (0.1g/U) for insulin sensitivity
- Set various presets to establish favourites, tag activities and personalise insulin delivery based on daily routine
ΩThe Pod has an IP28 rating for up to 7.6 metres (25 feet) for up to 60 minutes. The PDM is not waterproof.
1. In Automated Mode, SmartAdjust technology uses your total daily insulin (TDI) to set a new Adaptive Basal Rate for you.
2. Brown S. et al. Diabetes Care. 2021;44:1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6 - 70 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time in hyperglycaemic range (>10.0 mmol/L or >180mg/dL) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 28.9% vs. 22.8%; 44.8% vs 29.7%, P<0.0001, respectively. Mean time in hypoglycaemic range (<3.9 mmol/L or <70 mg/dL) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 2.89% vs. 1.32%, P<0.0001; 2.21% vs. 1.78, P=0.8153, respectively.
3. Sherr J. et al. Diabetes Care. 2022; 45:1907-1910. Single-arm multicenter clinical trial in 80 pre-school children (aged 2-5.9 yrs) with T1D. Study included a 14-day standard therapy (ST) phase followed by a 3-month AID phase with Omnipod 5 system. Mean time in hyperglycaemic range (>10.0 mmol/L or >180mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 39.4% vs. 29.5%, P<0.0001, respectively. Mean time in hypoglycaemic range (<3.9 mmol/L or <70 mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 3.43% vs. 2.46%, P=0.0204.
The Omnipod 5 Automated Insulin Delivery System is a single hormone insulin delivery system intended to deliver U-100 insulin subcutaneously for the management of type 1 diabetes in persons aged 2 and older requiring insulin. The Omnipod 5 System is intended for single patient use. The Omnipod 5 System is compatible with the following U-100 insulins: NovoLog®/NovoRapid®, Humalog®, Admelog®/Insulin lispro Sanofi®, Trurapi®/Insulin aspart Sanofi®, and Kirsty®. Refer to the Omnipod® 5 Automated Insulin Delivery System User Guide for complete safety information including indications, contraindications, warnings, cautions, and instructions. The Omnipod DASH® Insulin Management System is intended for subcutaneous delivery of insulin at set and variable rates for the management of diabetes mellitus in persons requiring insulin. Additionally, the Omnipod DASH® System permits manual entry of readings from blood glucose meters, enabling storage and display of blood glucose measurements.
The Omnipod® System and Omnipod DASH® System have been tested and found to be suitable for use with the following U-100 insulin: NovoLog®/NovoRapid®, Humalog®, Fiasp®, Lyumjev®, Admelog®/ Insulin lispro Sanofi® and Trurapi®/Truvelog®/Insulin aspart Sanofi®, Kirsty®, and Apidra®. Refer to the Omnipod DASH® Insulin Management System User Guides for complete safety information including indications, contraindications, warnings, cautions, and instructions. These User Guides are periodically updated. Visit Omnipod.com to see the most recent version and to find other helpful information. To access these User Guides in other languages, please visit Omnipod.com. DO NOT start using your system or change your settings without adequate training and guidance from your healthcare provider. Initiating and adjusting settings incorrectly can result in over-delivery or underdelivery of insulin, which could lead to hypoglycemia or hyperglycemia.
Omnipod Discover is a retrospective data analytics and reporting system intended for Omnipod 5 and Omnipod DASH System users or their caregivers and their healthcare providers for the analysis of glucose and insulin delivery data in home and healthcare settings. It is intended as supplemental data for the users to support diabetes management and aid healthcare providers in patient care. Omnipod Discover is not intended for people with diabetes in acute care settings or for real-time patient monitoring. The Omnipod Discover software platform is not intended to replace the primary real-time display of the sensor or insulin delivery data on the device, nor does it control any functions of the Omnipod System. Any medical treatment decision or adjustments should not be made based on this software platform; a qualified healthcare professional needs to be consulted to make such decisions.