Omnipod® 5

Omnipod 5 Automated Insulin Delivery (AID) System is a wearable, tubeless, hybrid closed loop system that integrates with the Dexcom G7, for people living with type 1 diabetes aged 2 years and older requiring insulin.
Omnipod 5 System: Three Simple Parts
Controller + Pod + Sensor


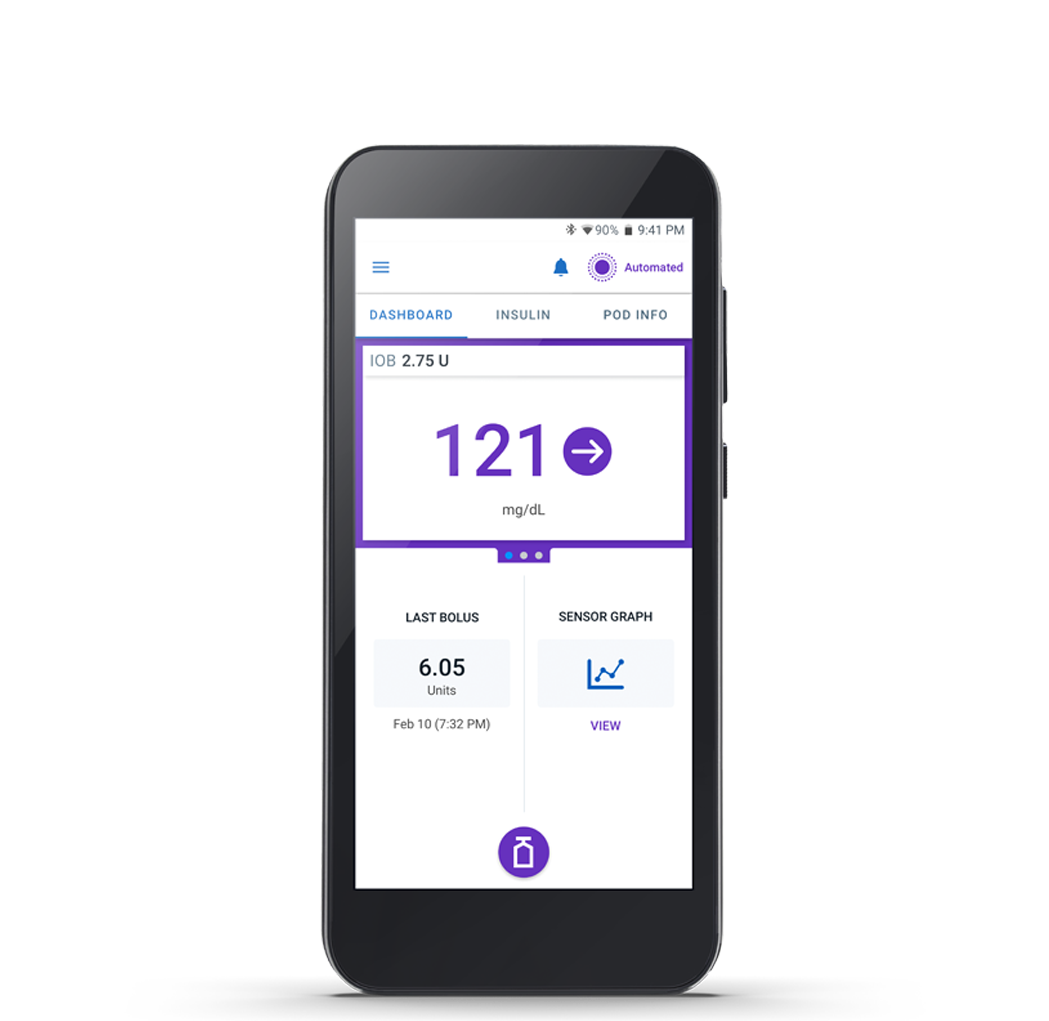
Omnipod 5 Controller
The Omnipod 5 Controller allows you to monitor and control the Pod using Bluetooth® wireless technology.



Pod
Tubeless, wearable, and waterproof,** the Pod, with built-in SmartAdjust™ technology, sits right on your patient’s body and automatically§ adjusts insulin delivery for up to 3 days or 72 hours.
**The Pod has an IP28 rating for up to 7.6 metres (25 feet) for up to 60 minutes. The Omnipod 5 Controller is not waterproof. Please consult sensor manufacturer user guide for sensor waterproof rating.
§Automated Mode requires compatible CGM Sensor
Sensor
CGM sensor continuously sends glucose values to the Pod, so your patients can get real-time data - without the finger pricks†.


Sensors shown without the necessary adhesive. They are sold and prescribed separately. Sensor availability may vary per country or region.
†Fingerpricks required for diabetes treatment decisions if symptoms or expectations do not match readings.
Omnipod 5’s Algorithm: SmartAdjust™ Technology
Predicts
Glucose 60 minutes into the future
Adjusts
Insulin delivery using the selected Target Glucose
Delivers
Insulin doses every 5 minutes (as needed)
Always adjusting, so you don’t have to.
Omnipod® 5 with SmartAdjust™ technology automatically adjusts to your patients’ personal needs by increasing, decreasing, or pausing insulin delivery every five minutes – which may help protect against highs and lows.1,2
Watch this video to learn more.
Improved Clinical Outcomes with Omnipod 5
Omnipod 5 improved glycaemic control in adults, adolescents and children with T1D in pivotal studies.1,2
76%
time in range (TIR) at a target of 6.1 mmol/L (110 mg/dL) in adults and adolescents (14–70 years) and 68% overall TIR in children (2- 13.9 years)1,2
HbA1c
was significantly reduced in very young children (2.0–5.9 years), children (6–13.9 years), adults and adolescents (14–70 years) by 0.5%, 0.7% and 0.4% (4.2, 6, and 7.8 mmol/mol) respectively1,2
33%
reduced time in hyperglycaemia in children and 24% in adults and adolescents1
60%
reduction in time in hypoglycaemia overnight and 46% overall in adults and adolescents1
Omnipod® 5 improved users’ quality of life in clinical studies3,4
- Parents of children using Omnipod 5 reported improvements in quality of life, sleep quality, confidence avoiding hypoglycemia, and mental well-being3,4
- Adults using Omnipod reported improvements in diabetes distress, stress eating, confidence avoiding hypoglycemia, and mental well-being3,4
1. Brown S. et al. Diabetes Care. 2021;44:1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6 - 70 yrs [Adults N=128, aged 14-70 years, Children N=112, aged 6-13.9 years). Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time in hyperglycaemic range (>10.0 mmol/L or >180mg/dL) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 28.9% vs. 22.8%; 44.8% vs 29.7%, P<0.0001, respectively. Mean time in hypoglycaemic range (<3.9 mmol/L or <70 mg/dL) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 2.89% vs. 1.32%, P<0.0001; 2.21% vs. 1.78, P=0.8153, respectively. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in children as measured by CGM: ST = 52.5%, 3-mo Omnipod 5 = 68.0%, P<0.0001. Mean HbA1c: ST vs. Omnipod 5 use in adults/adolescents and children, respectively (7.16% vs 6.78% or 55 mmol/mol vs. 51 mmol/mol, P<0.0001; 7.67% vs 6.99% or 60mmol/mol vs 53 mmol/mol), P<0.0001). Average time above range (>10 mmol/L or >180 mg/dL) in children: ST vs. 3-month Omnipod 5 = 45.3% vs. 30.2%. Change is relative comparison. Average time above range (>10 mmol/L or >180 mg/dL) in adults: ST vs Omnipod 5: 32.4% vs. 24.7%. Change is relative. Mean time in hypoglycaemic range (<3.9 mmol/L or <70mg/dL as measured by CGM; 12AM - < 6AM) in adults as measured by CGM: ST: 2.07% vs. Omnipod 5: 0.82%, P<0.0001 Comparison is a relative change.
2. Sherr J. et al. Diabetes Care. 2022; 45:1907-1910. Single-arm multicenter clinical trial in 80 pre-school children (aged 2-5.9 yrs) with T1D. Study included a 14-day standard therapy (ST) phase followed by a 3-month AID phase with Omnipod 5 system. Mean time in hyperglycaemic range (>10.0 mmol/L or >180mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 39.4% vs. 29.5%, P<0.0001, respectively. Mean time in hypoglycaemic range (<3.9 mmol/L or <70 mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 3.43% vs. 2.46%, P=0.0204. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in very young children (2 - 5.9 yrs) as measured by CGM: ST = 57.2%, 3-mo Omnipod 5 = 68.1%, P<0.0001. Mean HbA1c: ST vs. Omnipod 5 use: 7.4% vs 6.9% or 57 mmol/mol vs 53 mmol/mol, P<0.05.
3. Hood KK et al. Diabetes Obes Metab. 2024. Study of 81 children aged 6-11.9 with type 1 diabetes using standard therapy (ST) for 2 weeks followed by 3-months of the Omnipod 5 System. Mean caregiver self-reported Hypoglycemia Confidence Scale score ST vs Omnipod 5: 3.34 vs 3.59, p-value <0.0001, respectively. During the Omnipod 5 pivotal trial, parents of children aged 6-11.9 years (N=82) experienced an improvement in emotional distress levels and mental well-being survey scores after 3 months of Omnipod 5 use compared to standard therapy: mean P-PAID-C score = 40.7 vs. 47.4; mean WHO-5 score = 72.9 vs. 67.5, respectively. During the Omnipod 5 pivotal trial, adults aged 18-70 (N=115) experienced an improved diabetes distress survey score after 3 months of AID use: mean: 1.48 vs. 1.64 (P < 0.001). During the Omnipod 5 pivotal trial, parents of children aged 6-11.9 years (N=82) experienced an improvement in emotional distress levels and mental well-being survey scores after 3 months of Omnipod 5 use compared to standard therapy: mean P-PAID-C score = 40.7 vs. 47.4; mean WHO-5 score = 72.9 vs. 67.5, respectively.
During the Omnipod 5 pivotal trial, parents of children 6-11.9 years (N=82) experienced an improvement in sleep quality survey score after 3 months of Omnipod 5 use compared to standard therapy: mean PSQI Overall Sleep Quality Subscore = 0.70 vs 1.12, respectively.
4. Polonsky WH et al. Diabetes Res Clin Pract 2022;190:109998. Study of 115 adults aged 18-70 years with type 1 diabetes using standard therapy (ST) for 2 weeks followed by 3-months of the Omnipod 5 System. Mean self-reported Hypoglycemia Confidence Scale score ST vs Omnipod 5: 3.52 vs 3.65, p-value = 0.0002, respectively. Adults aged 18-70 (N=115) experienced reduction in eating distress score in T1-DDS survey after 3 months of Omnipod 5 use vs baseline: 1.73 vs. 1.97, (P=0.0003), respectively.
Initiating a Patient on Omnipod 5
Help your patients have a successful start on Omnipod® 5.
Diabetes data management, simplified
Experience a smarter way to support your Omnipod® 5 patients. With sensor data, insulin, carbs, usage metrics, and Pod settings all in one place—plus a seamless cloud connection, Omnipod Discover™ delivers simple diabetes data all in one report. It continually and automatically collects Omnipod 5 and sensor data wirelessly to help track glucose trends, insulin delivery data, and pump settings all in one report.
So you can optimize your time with your Omnipod 5 patients.
Omnipod 5 Resources for your Patients
All of the resources, all in one place. Visit our resources page for User Guides, How-to-Videos, FAQs and more.
Omnipod Discover is a retrospective data analytics and reporting system intended for Omnipod 5 or their caregivers and their healthcare providers for the analysis of glucose and insulin delivery data in home and healthcare settings. It is intended as supplemental data for the users to support diabetes management and aid healthcare providers in patient care. Omnipod Discover is not intended for people with diabetes in acute care settings or for real-time patient monitoring. The Omnipod Discover software platform is not intended to replace the primary real-time display of the sensor or insulin delivery data on the device, nor does it control any functions of the Omnipod System. Any medical treatment decision or adjustments should not be made based on this software platform; a qualified healthcare professional needs to be consulted to make such decisions.