Life gets bigger when diabetes management gets smaller. Simplify Life with Omnipod® 5!


The first and only tubeless automated insulin delivery system that works with the leading sensor brands*
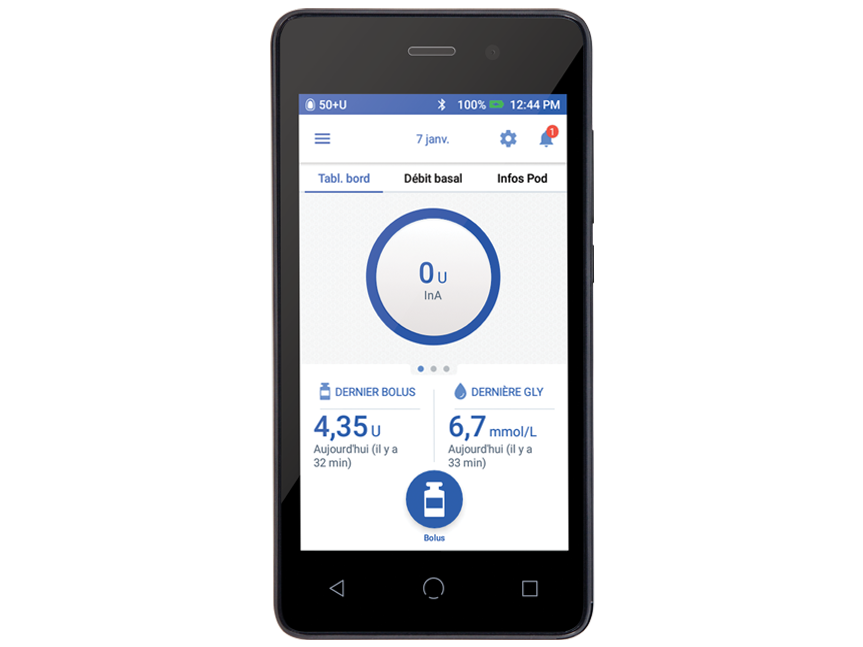
Omnipod 5 automatically adjusts insulin delivery every five minutes based on CGM readings, to help protect against highs and lows.1,2 It’s the system that never sleeps!
What is Pod Therapy?
Pod Therapy is simple, tubeless, discreet insulin pump therapy for people living with type 1 diabetes.
Each waterproof†, wearable Pod automatically adjusts insulin for up to three days (72 hours), controlled wirelessly by you, wherever you are.
With no multiple daily injections and no tubes, Pod Therapy is insulin pump therapy, simplified. Only found with Omnipod®.


Meet our tubeless, wearable Omnipod lineup
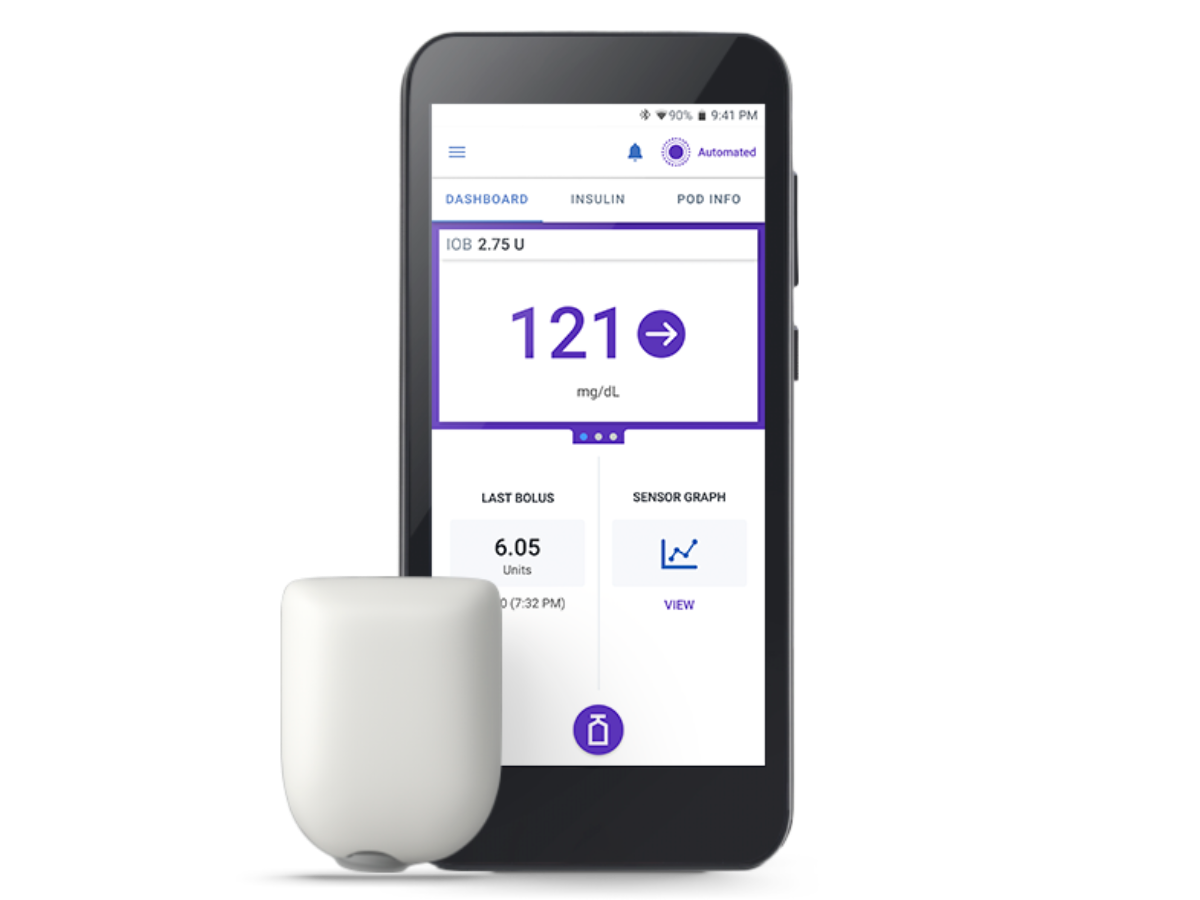

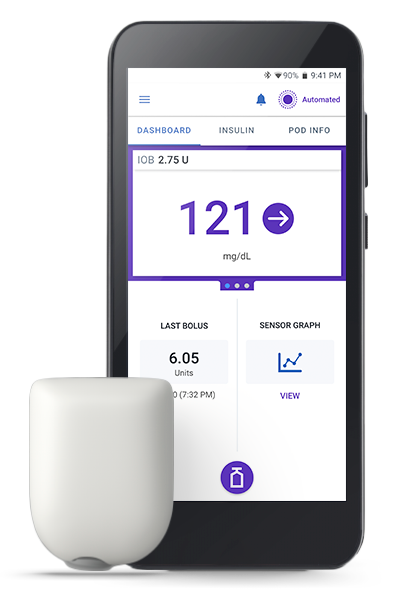
Omnipod 5 Automated Insulet Delivery System
Now compatible with Dexcom G6, Dexcom G7 and FreeStyle Libre 2 Plus sensors! Experience the freedom of automated insulin delivery and improved time in range1,2 with your preferred glucose sensor.
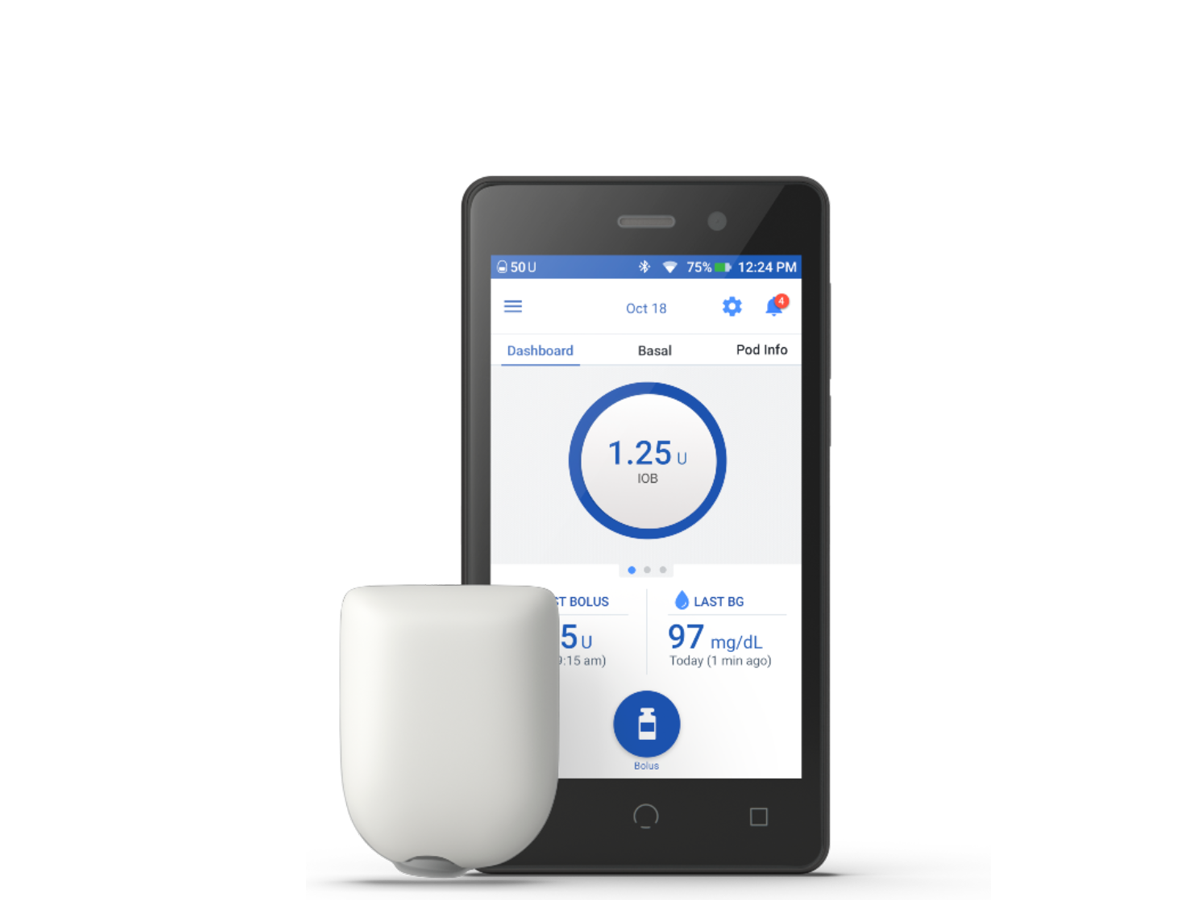

Omnipod DASH® Insulin Management System
You’re in control with the Omnipod DASH® Personal Diabetes Manager. Discover discreet, precise insulin dosing and customisable programmes designed to fit around your lifestyle.
How can I find out more about the Omnipod?
We are delighted to have Geffen Medical as our distributor in Israel.
Please contact Geffen Medical directly to learn how to get started on the Omnipod 5 or Omnipod DASH.
Adresse: HaYarkon 5B Street ,Bnei Brak, Israel, 5120125
E-mail: [email protected]
Telefon: +972-3-6900300/*6364
Here’s what our Podders® have to say about Omnipod…
Omnipod 5 has allowed me to get a good night sleep. That's the first time I can say that in a long time.
Alvin
Podder since 2017
It helps me feel like a normal kid, just with a little bit of help.
Romey T.
Sponsored Omnipod® User
& Podder since 2019
I don’t have to spend as much time thinking about diabetes.
Clare F.
Podder® since 2013
1. Brown S. et al. Diabetes Care. 2021;44:1630-1640. Prospective pivotal trial in 240 participants with T1D aged 6 - 70 yrs. Study included a 14-day standard therapy (ST) phase followed by a 3-month Omnipod 5 hybrid closed-loop (HCL) phase. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in adults/adolescents as measured by CGM: ST = 64.7%, 3-mo Omnipod 5 = 73.9%, P<0.0001. Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) in children as measured by CGM: ST = 52.5%, 3-mo Omnipod 5 = 68.0%, P<0.0001. Mean HbA1c: ST vs. Omnipod 5 use in adults/adolescents (14-70 yrs) and children (6-13.9 yrs), respectively (7.16% vs 6.78% or 55 mmol/mol vs. 51 mmol/mol, P<0.0001; 7.67% vs 6.99% or 60mmol/mol vs 53 mmol/mol), P<0.0001. Mean time >10.0 mmol/L or >180mg/dL (12AM-<6AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 32.1% vs. 20.7%; 42.2% vs 20.7%, P<0.0001, respectively. Mean time >10.0 mmol/L or >180mg/dL (6AM-<12AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 32.6% vs. 26.1%; 46.4% vs 33.4%, P<0.0001, respectively. Mean time <3.9 mmol/L or <70 mg/dL (12AM-<6AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 3.64% vs. 1.17%, P<0.0001; 2.51% vs. 1.78, P=0.0456, respectively. Mean time <3.9 mmol/L or <70 mg/dL (6AM-<12AM) as measured by CGM in adults/adolescents and children ST vs. 3-mo Omnipod 5: 2.64% vs. 1.37%, P<0.0001; 2.13% vs. 1.98%, P=0.2545, respectively.
2. Sherr J. et al. Diabetes Care. 2022; 45:1907-1910. Single-arm multicenter clinical trial in 80 pre-school children (aged 2-5.9 yrs) with T1D. Study included a 14-daystandard therapy (ST) phase followed by a 3-month AID phase with Omnipod 5 system. Mean HbA1c as measured in very young children, ST vs. Omnipod 5 use:7.4% vs 6.9% or 57 mmol/ml vs. 53 mmol/mol; (P<0.0001). Mean time in range (3.9-10.0 mmol/L or 70-180mg/dL) as measured by CGM in children ST vs. 3-mo Omnipod 5: 57.2% vs 68.1%, P<0.0001. Mean time >10.0 mmol/L or >180mg/dL (12AM-<6AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 38.4% vs. 16.9%, P<0.0001, respectively. Mean time >10.0 mmol/L or >180mg/dL (6AM-<12AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 39.7% vs. 33.7%, P<0.0001, respectively. Mean time <3.9 mmol/L or <70 mg/dL (12AM-<6AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 3.41% vs. 2.13%, P=0.0185. Mean time <3.9 mmol/L or <70 mg/dL (6AM-<12AM) as measured by CGM in children ST vs. 3-mo Omnipod 5: 3.44% vs. 2.57%, P=0.0799.
A separate prescription is required for the sensor. The Dexcom G6, Dexcom G7 and FreeStyle Libre 2 Plus sensors are sold separately.
*Requires Dexcom G6, Dexcom G7 or FreeStyle Libre 2 Plus sensor. Bolus for meals and corrections are still needed.
† The Pod has an IP28 rating for up to 7.6 metres (25 feet) for 60 minutes. The Omnipod 5 Controller is not waterproof. Please see sensor manufacturer user guide for sensor waterproof rating‡ Finger pricks required for diabetes treatment decisions if symptoms or expectations do not match readings.